Here are the essential concepts you must grasp in order to answer the question correctly.
Alkyl Halides
Alkyl halides are organic compounds containing a carbon atom bonded to a halogen atom (F, Cl, Br, I). They serve as versatile intermediates in organic synthesis, allowing for various reactions such as nucleophilic substitutions and eliminations. Understanding their reactivity is crucial for manipulating them to form desired products, such as ethers or alcohols.
Recommended video:
How to name alkyl halides
Nucleophilic Substitution Reactions
Nucleophilic substitution reactions involve the replacement of a leaving group (like a halogen) in an alkyl halide with a nucleophile. This process can occur via two main mechanisms: SN1 (unimolecular) and SN2 (bimolecular). The choice of mechanism depends on factors such as the structure of the alkyl halide and the strength of the nucleophile, which is essential for synthesizing compounds like 2-methoxybutane.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Ether Synthesis
Ether synthesis often involves the reaction of an alkyl halide with an alcohol or an alkoxide ion. In the case of preparing 2-methoxybutane, an alkoxide derived from methanol can act as a nucleophile to attack the alkyl halide, leading to the formation of the ether. Understanding the conditions and reagents required for this transformation is key to successfully synthesizing ethers in organic chemistry.
Recommended video:
The Mechanism of Williamson Ether Synthesis.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: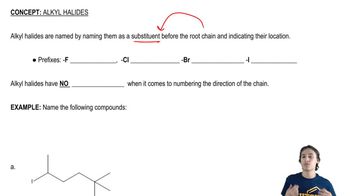



 8:33m
8:33m