Here are the essential concepts you must grasp in order to answer the question correctly.
Chair Conformation
The chair conformation is a three-dimensional representation of cyclohexane that minimizes steric strain and torsional strain. It features alternating axial and equatorial positions for substituents, allowing for more stable arrangements. Understanding this conformation is crucial for visualizing how substituents affect the molecule's overall stability and reactivity.
Recommended video:
What is a chair conformation?
Planar Representation
Planar representation refers to the two-dimensional depiction of a molecule, which simplifies the visualization of its structure. When converting a chair conformation to a planar form, it is essential to represent the bonds and angles accurately to maintain the integrity of the molecular geometry. This representation helps in understanding the spatial arrangement of atoms and substituents.
Recommended video:
Rules for Predicting Planarity
Axial and Equatorial Positions
In the chair conformation of cyclohexane, substituents can occupy either axial or equatorial positions. Axial substituents are oriented perpendicular to the plane of the ring, while equatorial substituents are oriented parallel to the plane. The choice of position significantly influences steric interactions and the overall stability of the molecule, making it a key factor in conformational analysis.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution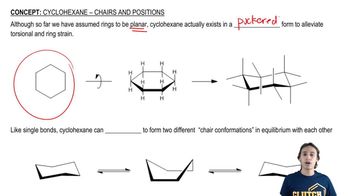



 1:18m
1:18m