Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Mechanisms
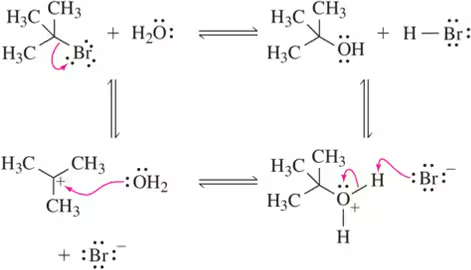
Reaction mechanisms describe the step-by-step process by which reactants are converted into products. Understanding these mechanisms is crucial for predicting the outcome of a reaction, including the reverse reaction. Each step involves the breaking and forming of bonds, and the movement of electrons, which can be illustrated using curved arrows in organic chemistry.
Recommended video:
Reversibility of Reactions
Many chemical reactions are reversible, meaning that the products can react to regenerate the original reactants. The extent to which a reaction can proceed in either direction is influenced by factors such as temperature, concentration, and the presence of catalysts. Recognizing the conditions that favor the reverse reaction is essential for accurately drawing its mechanism.
Recommended video:
Curved Arrow Notation
Curved arrow notation is a visual representation used in organic chemistry to depict the movement of electrons during chemical reactions. Arrows indicate the direction of electron flow, showing how bonds are formed and broken. Mastery of this notation is vital for illustrating both forward and reverse reaction mechanisms clearly and accurately.
Recommended video:
Alternative MO Notation for Dienes
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 5:14m
5:14m