Here are the essential concepts you must grasp in order to answer the question correctly.
Williamson Ether Synthesis
Williamson Ether Synthesis is a method for creating ethers through the reaction of an alkoxide ion with a primary alkyl halide. This reaction involves nucleophilic substitution, where the alkoxide acts as a nucleophile, attacking the electrophilic carbon of the alkyl halide, leading to the formation of an ether. The choice of reactants is crucial, as steric hindrance can affect the reaction's success.
Recommended video:
The Mechanism of Williamson Ether Synthesis.
Alkyl Halides
Alkyl halides are organic compounds containing a carbon atom bonded to a halogen atom (such as chlorine, bromine, or iodine). They serve as electrophiles in nucleophilic substitution reactions, making them essential reactants in ether synthesis. The structure of the alkyl halide, particularly whether it is primary, secondary, or tertiary, influences the reaction pathway and the feasibility of the Williamson Ether Synthesis.
Recommended video:
How to name alkyl halides
Nucleophiles
Nucleophiles are species that donate an electron pair to form a chemical bond in a reaction. In the context of ether synthesis, nucleophiles such as alkoxides or amines are crucial as they attack the electrophilic carbon of alkyl halides. The strength and structure of the nucleophile can significantly affect the reaction rate and the selectivity of the ether formation.
Recommended video:
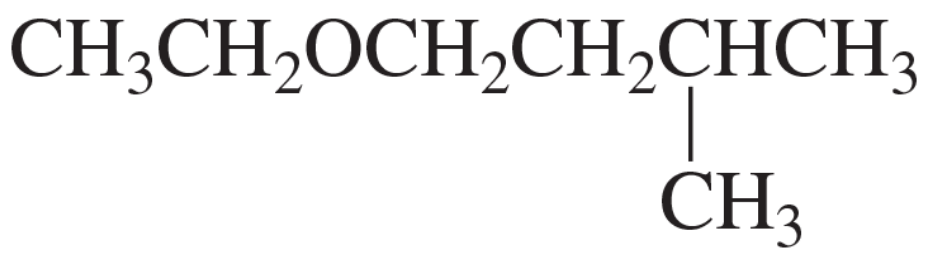
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:50m
3:50m