Textbook Question
Draw the most stable conformation of
c. cis-1-tert-butyl-4-isopropylcyclohexane.
1585
views
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution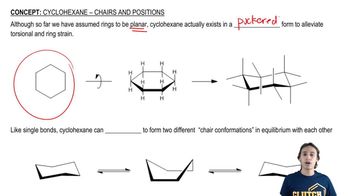



 4:02m
4:02mMaster Equatorial Preference with a bite sized video explanation from Johnny Betancourt
Start learning