Here are the essential concepts you must grasp in order to answer the question correctly.
Saponification
Saponification is a chemical reaction that involves the hydrolysis of an ester in the presence of a base, typically resulting in the formation of an alcohol and a carboxylate salt. This reaction is crucial in organic chemistry, particularly in the production of soaps from fats and oils. The rate of saponification can be influenced by the structure of the ester, including steric hindrance and electronic effects.
Recommended video:
Electronic Effects
Electronic effects refer to the influence of substituents on the reactivity of a molecule due to their ability to donate or withdraw electron density. In the case of methyl p-methoxybenzoate, the methoxy group is an electron-donating group that can stabilize the transition state during saponification, potentially increasing the reaction rate compared to methyl benzoate, which lacks this stabilizing effect.
Recommended video:
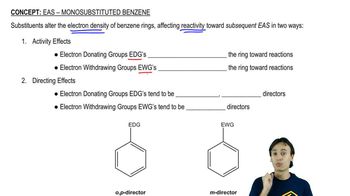
Activity and Directing Effects
Steric Hindrance
Steric hindrance occurs when the spatial arrangement of atoms within a molecule prevents certain reactions from occurring efficiently. In the context of saponification, bulky substituents near the ester bond can impede the approach of the nucleophile (usually hydroxide ion), slowing down the reaction. Understanding the steric effects of substituents is essential for predicting the reactivity of different esters.
Recommended video:
Understanding steric effects.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: