Here are the essential concepts you must grasp in order to answer the question correctly.
Sigmatropic Rearrangements
Sigmatropic rearrangements are a type of pericyclic reaction where a sigma bond and a pi system undergo a concerted transformation. These reactions can be classified based on the number of atoms involved in the migration, such as [1,3] sigmatropic migrations, which involve the migration of a substituent across a three-atom bridge. Understanding the mechanism of these rearrangements is crucial for analyzing their feasibility under different conditions.
Recommended video:
Nomenclature of Sigmatropic Shifts
Thermal vs. Photochemical Conditions
Thermal conditions refer to reactions occurring at elevated temperatures, where the energy provided can facilitate bond breaking and formation. In contrast, photochemical conditions involve the absorption of light, which can provide the necessary energy to promote certain reactions that are otherwise unfavorable under thermal conditions. The distinction between these conditions is essential for understanding why certain migrations are allowed or disallowed.
Recommended video:
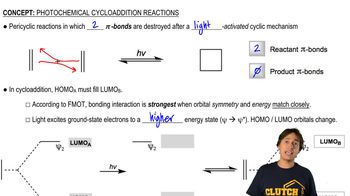
MO Theory of Photochemical Cycloadditions
Orbital Symmetry and Conservation
Orbital symmetry plays a critical role in determining the feasibility of pericyclic reactions. According to the Woodward-Hoffmann rules, the conservation of orbital symmetry must be maintained during a reaction. For [1,3] sigmatropic migrations of hydrogen, the symmetry requirements under thermal conditions are not satisfied, making these migrations forbidden, while carbon migrations can occur due to favorable symmetry interactions.
Recommended video:
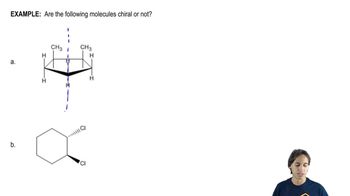
Determining Chirality with Plane of Symmetry
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: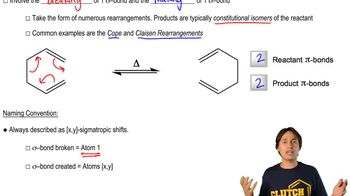

 3:51m
3:51m