Here are the essential concepts you must grasp in order to answer the question correctly.
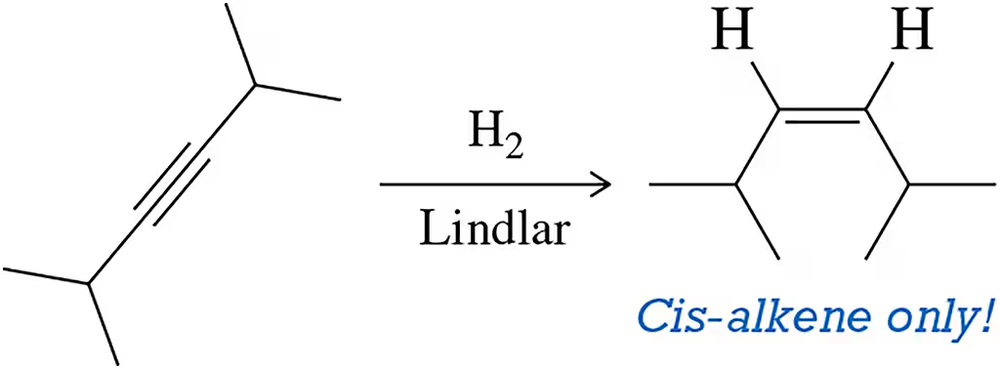
Lindlar Catalyst
The Lindlar catalyst is a palladium-based catalyst that is partially poisoned with lead salts. This modification allows it to selectively hydrogenate alkynes to cis-alkenes without further reducing them to alkanes. The controlled reactivity of the Lindlar catalyst is crucial for achieving the desired stereochemistry in the reduction process.
Recommended video:
Introduction to Catalysis Concept 1
Stereochemistry of Alkenes
Stereochemistry refers to the spatial arrangement of atoms in molecules and how this affects their chemical properties. In the case of alkenes, the cis and trans configurations arise from the restricted rotation around the double bond. The use of the Lindlar catalyst specifically leads to the formation of the cis-alkene due to the catalyst's ability to stabilize the transition state that favors this configuration.
Recommended video:
Polymer Stereochemistry Concept 1
Selective Hydrogenation
Selective hydrogenation is a chemical process that adds hydrogen to unsaturated compounds, such as alkynes, while controlling the extent of the reaction. In the context of the Lindlar catalyst, this process is designed to stop at the alkene stage, preventing complete reduction to alkanes. This selectivity is essential for producing specific products with desired properties, such as the cis-alkene in this reaction.
Recommended video:
The definition of hydrogenation.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 0:48m
0:48m