Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It relies on the magnetic properties of certain nuclei, such as hydrogen-1 (1H), to provide information about the number and environment of hydrogen atoms in a molecule. The resulting spectrum displays signals that correspond to different hydrogen environments, allowing chemists to infer structural details.
Recommended video:
Chemical Environment
The chemical environment refers to the specific surroundings of a hydrogen atom within a molecule, which can influence its magnetic resonance signal. Factors such as electronegativity of nearby atoms, hybridization, and molecular symmetry affect the chemical shift observed in the NMR spectrum. Identifying unique environments helps predict the number of distinct signals in the spectrum.
Recommended video:
Chemical Reactions of Phosphate Anhydrides Concept 1
Isomerism
Isomerism is the phenomenon where compounds share the same molecular formula but differ in structure or spatial arrangement. For the molecular formula C6H14, various structural isomers exist, such as straight-chain and branched alkanes. Each isomer can produce a different number of NMR signals due to variations in hydrogen environments, making it essential to consider isomerism when analyzing NMR data.
Recommended video:
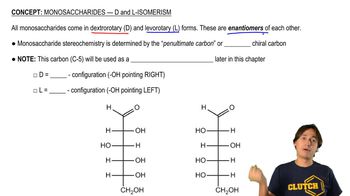
Monosaccharides - D and L Isomerism
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:35m
4:35m