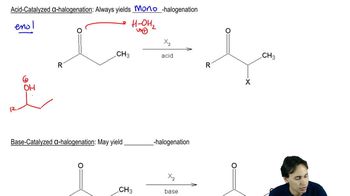
Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Catalyzed Reactions
Acid-catalyzed reactions involve the use of an acid to increase the rate of a chemical reaction. In organic chemistry, acids can protonate nucleophiles or electrophiles, enhancing their reactivity. This mechanism is crucial in the formation of acetals, where the acid facilitates the reaction between a carbonyl compound and an alcohol by activating the carbonyl carbon.
Recommended video:
Formation of Acetals
The formation of acetals occurs when a carbonyl compound, such as a ketone or aldehyde, reacts with an alcohol in the presence of an acid catalyst. This process involves the nucleophilic attack of the alcohol on the carbonyl carbon, followed by the elimination of water and the formation of a stable acetal. Understanding this mechanism is essential for predicting the products of the reaction between cyclohexanone and ethylene glycol.
Recommended video:
Nucleophilic Attack
Nucleophilic attack is a fundamental concept in organic chemistry where a nucleophile, which is an electron-rich species, attacks an electrophile, an electron-deficient species. In the context of the reaction between cyclohexanone and ethylene glycol, the hydroxyl group of ethylene glycol acts as a nucleophile, attacking the electrophilic carbonyl carbon of cyclohexanone, leading to the formation of the acetal.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: