Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It exploits the magnetic properties of certain nuclei, such as hydrogen (1H) and carbon (13C), to provide information about the number of atoms, their environment, and connectivity. In proton-coupled 13C NMR, the interactions between protons and carbon atoms are observed, revealing details about the molecular structure.
Recommended video:
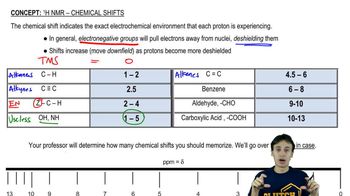
Chemical Shifts
Chemical shifts in NMR spectroscopy refer to the variation in resonance frequency of a nucleus due to its electronic environment. In 13C NMR, chemical shifts are measured in parts per million (ppm) and provide insights into the types of carbon environments present in a molecule. For 2-chloroethanol, the chemical shifts will reflect the presence of the hydroxyl group and the chlorine atom, influencing the electronic environment of the carbon atoms.
Recommended video:
Multiplicity and Coupling Patterns
Multiplicity in NMR refers to the number of peaks observed for a particular signal, which is influenced by the number of neighboring protons (n) according to the n+1 rule. In the case of 2-chloroethanol, the coupling patterns will reveal how the protons on adjacent carbons interact, leading to distinct splitting of the carbon signals in the 13C NMR spectrum. Understanding these patterns is crucial for interpreting the spectrum and deducing the molecular structure.
Recommended video:
Common Splitting Patterns
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 4:m
4:m