Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It relies on the magnetic properties of certain nuclei, such as hydrogen (1H) and carbon (13C), to provide information about the environment surrounding these nuclei. The resulting spectrum displays peaks that correspond to different chemical environments, allowing chemists to infer structural details about the molecule.
Recommended video:
Deuterium Exchange
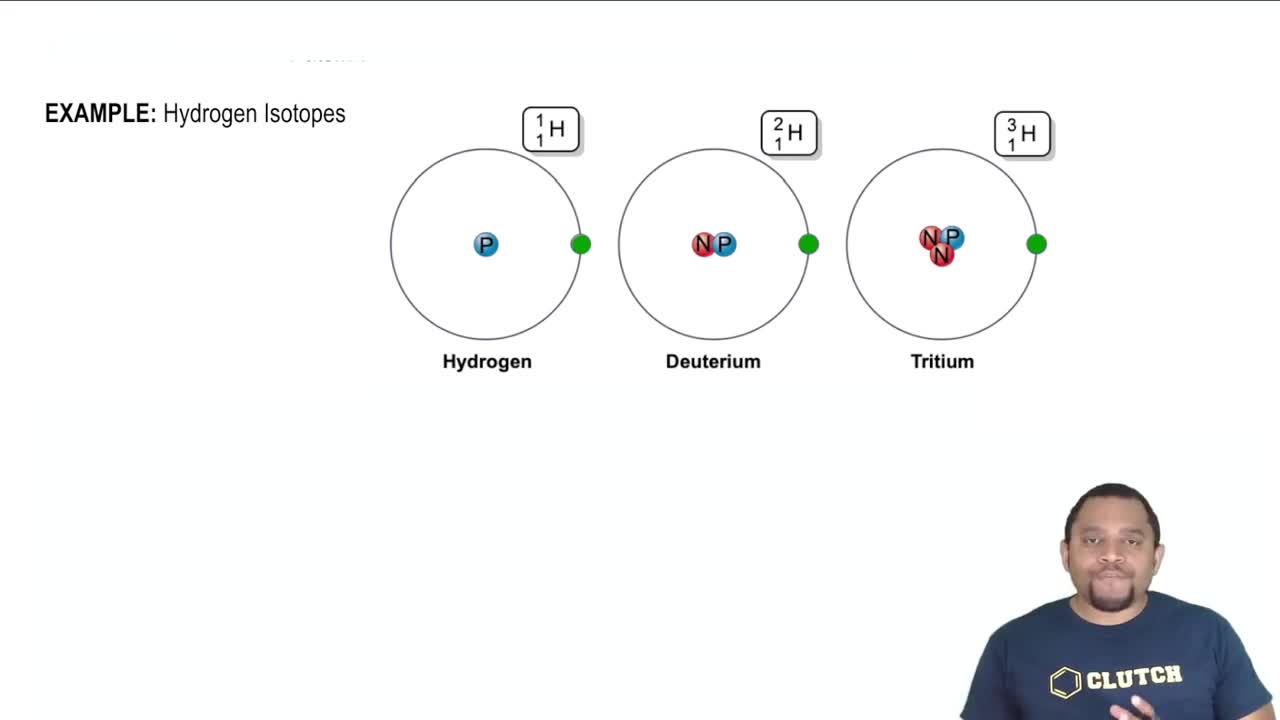
Deuterium exchange occurs when a hydrogen atom in a molecule is replaced by a deuterium atom (D), which is an isotope of hydrogen. In the context of ethanol shaken with D2O, the hydroxyl (–OH) hydrogen can exchange with deuterium, leading to the formation of deuterated ethanol. This exchange affects the NMR spectrum by altering the number and position of peaks, particularly in the region corresponding to the hydroxyl group.
Recommended video:
Understanding the hydrogen isotopes.
Chemical Shift
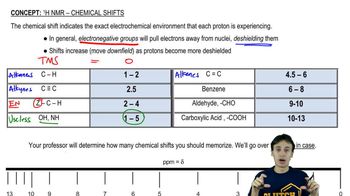
Chemical shift refers to the variation in the resonance frequency of a nucleus due to its electronic environment. In NMR spectroscopy, different functional groups and molecular environments cause shifts in the position of peaks on the spectrum. For ethanol, the chemical shifts of the protons in the –OH, –CH2, and –CH3 groups will be distinct, and the exchange with deuterium will lead to a noticeable change in the peak corresponding to the hydroxyl proton.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: