Here are the essential concepts you must grasp in order to answer the question correctly.
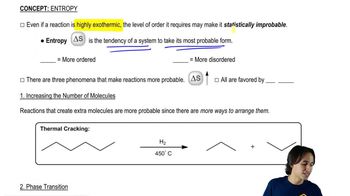
Entropy (∆S°)
Entropy, denoted as ∆S°, is a measure of the disorder or randomness in a system. In thermodynamics, a positive change in entropy (∆S° > 0) indicates an increase in disorder, while a negative change (∆S° < 0) suggests a decrease in disorder. Understanding how molecular arrangements and states of matter affect entropy is crucial for predicting the sign of ∆S° in various processes.
Recommended video:
Explaining what entropy is.
Phase Changes
Phase changes, such as melting, boiling, or sublimation, significantly influence entropy. For example, when a solid melts into a liquid, the molecules become more disordered, leading to an increase in entropy (∆S° > 0). Conversely, when a gas condenses into a liquid, the molecules become more ordered, resulting in a decrease in entropy (∆S° < 0). Recognizing the phase of substances involved in a process is essential for determining the sign of ∆S°.
Recommended video:
Merrifield Solid-Phase Peptide Synthesis Concept 1
Molecular Complexity
The complexity of molecules, including the number of atoms and the types of bonds, affects entropy. More complex molecules with greater degrees of freedom (e.g., rotational and vibrational modes) typically have higher entropy than simpler molecules. When analyzing a process, considering the molecular structures and their interactions can help predict whether the overall entropy change will be positive or negative.
Recommended video: