Write the molecular formula for a substance that contains two oxygen molecules, two carbons, and four hydrogen atoms. Be sure to follow the standard conventions of writing molecular formulas.
In each of the following reactions, identify the products and reactants, and state what class of reaction is shown. <IMAGE>
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Reactants and Products

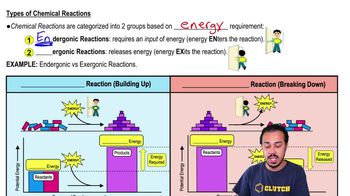
Types of Chemical Reactions
Balancing Chemical Equations

Indicate the true statements and then correct the false statements so that they are true.
a. Isotopes are atoms with differing numbers of protons and the same number of
neutrons.
b. A cation is a positive ion.
c. Redox reactions create ions.
d. Equal sharing of electrons leads to polar covalent bonds.
e. Ions are charged atoms.
f. CO2 is an inorganic molecule.
g. Isomers have the same molecular formula but different structures.
h. Adding a base to a solution will decrease the pH.
Fill in the blanks:Acids donate _______________________ to an aqueous solution, which will lead to a(n) _______________________ in pH. In contrast, bases donate _______________________ to an aqueous solution and will _______________________ the pH. The pH of a solution with more OH−
than H+ will have a(n) _______________________ pH.
