Here are the essential concepts you must grasp in order to answer the question correctly.
Atomic Number
The atomic number of an element is the number of protons found in the nucleus of an atom of that element. It uniquely identifies the element and determines its position on the periodic table. For example, lithium (Li) has an atomic number of 3, indicating it has three protons.
Recommended video:
Atomic Mass
Atomic mass is the weighted average mass of an element's isotopes, measured in atomic mass units (amu). It reflects the total number of protons and neutrons in the nucleus. For instance, oxygen (O) has an atomic mass of approximately 16 amu, which accounts for its most common isotopes.
Recommended video:
Chemical Symbol
A chemical symbol is a one- or two-letter notation used to represent an element on the periodic table. Each symbol is unique to an element, often derived from its English or Latin name. For example, potassium is represented by the symbol 'K', which comes from its Latin name 'kalium'.
Recommended video:
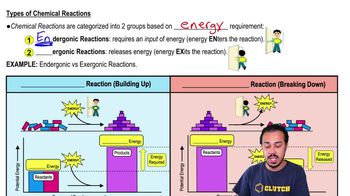
Types of Chemical Reactions
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: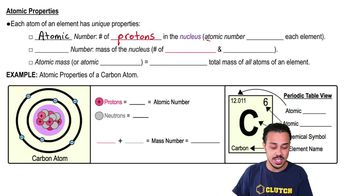


 5:05m
5:05m