Electron affinity, which is abbreviated as EA, represents the energy released from the addition of an electron to a gaseous atom or ion in kilojoules. So here, let's say we have carbon gas. We're dealing with a gaseous atom. We need to add an electron to it. Adding an electron means that it will be a reactant. The carbon absorbs that electron and gains a negative charge because electrons are negative. Now, in order to connect them together, energy has to be released. This energy represents the electron affinity. Here, it has to be a product because it's being released. Now, in chemistry, of course, there are exceptions that arise sometimes, and this is one of them. With electron affinity, the exception is we have electron affinities that are less than or equal to 0, and that happens when the element will not readily accept an electron. But why would an element want to accept an electron? Well, they may have a uniquely stable electron configuration or arrangement, i.e., the noble gases. We've talked about noble gases being perfect. If you're perfect in terms of your number of electrons, you don't have a need to accept another electron. So, the noble gases are a great example of elements that will not readily accept an electron. Now the general trend is electron affinity increases as we move from left to right across a period and going up a group. What does it mean that an electron can easily accept an electron or not? Well, we're going to say here that if the lower your electron affinity, then the electron will not easily be accepted. So, the smaller your electron affinity is, the harder it is for that element or ion to accept an electron. And we're going to say here that the greater your electron affinity is, then the more readily that ion or atom will accept an electron.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Periodic Trend: Electron Affinity (Simplified) - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIElectron affinity (ea) measures the energy released when an electron is added to a gaseous atom or ion. Generally, electron affinity increases across a period and decreases down a group. Noble gases exhibit electron affinities of less than or equal to 0 due to their stable electron configurations. Other exceptions include nitrogen and alkaline earth metals, which also resist accepting electrons. Understanding these trends is crucial for predicting an element's reactivity and stability in chemical reactions.
Electron Affinity is the energy released from the addition of an electron to a gaseous atom or ion.
Electron Affinity
Periodic Trend: Electron Affinity (Simplified) Concept 1
Video transcript
Periodic Trend: Electron Affinity (Simplified) Concept 2
Video transcript
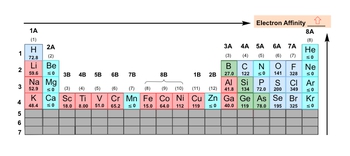
Electron affinity is not as clean-cut as other periodic trends. There are exceptions that pop up all over the place. Even more so with rules 5, 6, and 7, so much so that you really don't need to pay attention to them. Now realize here that as we're heading towards the top right corner, electron affinity more or less will be increasing. Also remember that when it comes to the noble gases, they're electronically perfect. They don't want additional electrons, so their electron affinities are less than or equal to 0. We also see this trend pop up with nitrogen, zinc, manganese, and in group 2a, beryllium, magnesium, and calcium. Because of their electron arrangements, they're also electronically stable, not perfect, just stable, and they don't readily accept an electron. So again, the general trend is as we're heading towards the top right corner, electron affinity increases. Noble gases don't ever want to accept an electron, and then these other ones that I've circled also fit this idea of being electronically stable. So, keep this in mind when you're looking at electron affinity.
Generally, electron affinity increases moving from left to right across a period and going up a group.
Periodic Trend: Electron Affinity (Simplified) Example 1
Video transcript
So here, let's take a look at this example question, and I've left the periodic table here just to help us. It says, which of the following halogens will release the most energy with the addition of an electron? So, remember, the general trend is as we're heading up towards a group, electron affinity more or less will be increasing. Now, if we take a look here, we have sulfur, neon, nitrogen, astatine, and bromine. Alright. So first of all, a halogen is in group 7A. These are not even halogens. They're not in group 7A. The answer is going to be either d or e. Realize here that I don't even show astatine. It's in one of the rows that we ignore because it's very unpredictable in terms of electron affinity, which means that it wouldn't be a viable option. So e will be our answer here through the process of elimination. Now, of course, there are exceptions to electron affinity. It's all over the place. We can see that the general trend should be as we go up a group, your electron affinity increases. But you can see that actually, chlorine has a slightly higher electron affinity than fluorine. But here, we don't have to worry about that because I wasn't asking about either one of those halogens. It was between bromine and astatine instead. But just remember, the general trend is as we head up a group, electron affinity more or less will be increasing.
Determine which atom in the following set has the largest electron affinity:N, O, C, B, Ne
a) N b) O c) C d) B e) Ne
Rank the following elements in order of increasing electron affinity:Cs, Hg, F, S
Which one of the following atoms has the least tendency to gain another electron?
Here’s what students ask on this topic:
What is electron affinity and how is it measured?
Electron affinity (EA) is the energy released when an electron is added to a gaseous atom or ion. It is measured in kilojoules per mole (kJ/mol). For example, when a carbon atom in the gas phase gains an electron, it releases energy, which is the electron affinity. The general trend is that electron affinity increases across a period (left to right) and decreases down a group (top to bottom) in the periodic table. Elements with high electron affinity readily accept electrons, while those with low or negative electron affinity, like noble gases, do not.
 Created using AI
Created using AIWhy do noble gases have electron affinities less than or equal to 0?
Noble gases have electron affinities less than or equal to 0 because they possess a stable electron configuration. Their outer electron shells are fully filled, making them electronically perfect. As a result, they have no tendency to gain additional electrons, which would disrupt their stable configuration. This stability is why noble gases are generally unreactive and do not readily accept electrons, resulting in low or negative electron affinities.
 Created using AI
Created using AIHow does electron affinity change across a period and down a group?
Electron affinity generally increases across a period from left to right and decreases down a group from top to bottom in the periodic table. As you move across a period, atoms have a greater nuclear charge, which attracts additional electrons more strongly, increasing electron affinity. Conversely, as you move down a group, the added electron is further from the nucleus due to increased atomic size, reducing the attraction and thus decreasing electron affinity.
 Created using AI
Created using AIWhat are some exceptions to the general trend of electron affinity?
While the general trend is that electron affinity increases across a period and decreases down a group, there are notable exceptions. Noble gases have electron affinities less than or equal to 0 due to their stable electron configurations. Other exceptions include nitrogen, zinc, manganese, and alkaline earth metals like beryllium, magnesium, and calcium. These elements have stable electron arrangements and do not readily accept additional electrons, resulting in lower electron affinities than expected.
 Created using AI
Created using AIWhy is understanding electron affinity important in chemistry?
Understanding electron affinity is crucial in chemistry because it helps predict an element's reactivity and stability in chemical reactions. Elements with high electron affinity are more likely to gain electrons and form negative ions, making them more reactive. Conversely, elements with low or negative electron affinity are less likely to gain electrons, making them less reactive. This knowledge is essential for predicting how elements will behave in various chemical processes and for designing reactions in both academic and industrial settings.
 Created using AI
Created using AI