Before we start naming acids, let's first talk about what exactly is an acid. Now, an acid is usually a covalent compound beginning with a hydrogen ion. So, the hydrogen ion in this case is H+ and another name for this hydrogen ion is called the hydronium ion. Now, going back to covalent, what exactly is meant by covalent compound? Well, a covalent compound is a compound that contains only nonmetals bonded together. Now, if you don't remember who the nonmetals are, make sure you go back and take a look at my videos on the periodic table and the classifications of the elements that lie within it. If we take a look here, some common types of acids, all of them are covalent because all of them possess nonmetals together. And since they're all covalent, we also see next that they all begin with hydrogen. That tells us that they all represent acids. So HCl, HN32, H2SO4, we see that some of them are pretty simple like HCl and some of them are pretty complicated like H3PO4. We'll talk about the different types of acids later on and the rules associated with naming them. Now, remember, we said that they usually begin with the hydrogen ion. "Usually" does not mean "always." And in chemistry, you're going to see that exceptions do pop up here and there. A good exception to this is acetic acid. Now, acetic acid can be drawn two different ways. The first way goes along with the definition we have. It starts with the hydrogen and is covalent because it only has nonmetals. But another way to write acetic acid is CH3COOH. In this case, the hydrogen ion is actually written in the back. Okay. So, this is an exception to our definition, where this is still an acid, but it doesn't begin with the hydrogen ion. Now that we know the basic structure of an acid, let's move on to some videos and let's tackle the different types of acids that exist and the ways of naming them.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Naming Acids - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIAcids are covalent compounds that typically begin with a hydrogen ion (H+), forming hydronium ions in solution. Binary acids consist of hydrogen and a nonmetal anion, while oxyacids contain hydrogen bonded to a polyatomic ion with oxygen. Naming binary acids involves the prefix "hydro," the base name of the nonmetal, and the suffix "ic acid." For oxyacids, if the polyatomic ion ends in "ate," it changes to "ic acid," and if it ends in "ite," it changes to "ous acid." Understanding these rules is essential for proper acid nomenclature.
Acids, for the most part, are covalent compounds that begin with a hydrogen.
Naming Acids
Naming Acids
Video transcript
Naming Acids
Video transcript
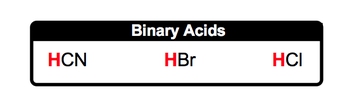
So here, let's take a look at binary acids. Now binary acids, since they're acids, are also represented as covalent compounds, and they contain the H+ ion, which is our hydrogen ion. But here, they are going to be bonded to a nonmetal anion that is not oxygen. Binary acids are covalent compounds, usually beginning with hydrogen, that contain no oxygen. Here, how do we form them? Well, we know that they possess the H+ ion, and let's think of a nonmetal they can be connected to in the anion form, which means they have a negative charge. So let's say we're dealing with iodine. Iodine is in group 7A, so its charge is minus 1. So here we have plus 1 from the hydrogen, minus 1 from the iodine. If you've seen our videos on writing ionic compounds, we apply the same principles here. Remember, if the numbers in the charges are the same, they simply cancel out, and you combine together your elements. So here, this would be HI. HI would represent a typical binary acid.
Alright, so here, rules for naming these binary acids. Well, step 1 is the prefix they are going to use. The prefix they use is hydro, and that basically represents the H+ ion. Then we're going to say step 2: use the base name of the nonmetal. Now here's the thing. Remember, the base name is usually the beginning part of the name of the nonmetal that is being used, and it's unchanged, except when we use sulfur or phosphorus. So, in their acid forms, for sulfur, we actually use the entire name. We don't use the base name. We use the entire name for sulfur. And for phosphorus, we don’t just use its base name; we use a little bit more. The base name of phosphorus is phosph, but in acid form, we actually use 'or' as well. So, again, the nonmetal part of the acid, that is not the H+, we use its base name except for sulfur and phosphorus. We use a little bit more than just their base names. Then finally, to end the name of the binary acid, we use "ic acid" for the end of the name. So we apply these principles, and we'll be able to name any type of binary acid.
Binary Acids contain a hydrogen ion connected to a nonmetal that is not oxygen.
Naming Acids Example 1
Video transcript
So for this example question, it says write the formula for each of the following compounds. We have hydroiodic acid, hydroselenic acid, and hydrofluoric acid. Alright. So we're going to say that hydro, we know that represents the H+ ion involved, so we have H+ for all of these. Since they're all binary acids, we know they all end with -ic acid. What we look at is the base name. The base name tells us the nonmetal involved. Iodine means we're dealing with iodine. It's in group 7A, so it's -1. Selenium is in group 6A, so it's -2. And then fluor means we're dealing with fluorine which is in group 7A, so it is -1. Now for the first one, for a, remember, when the numbers and the charges are the same, they simply cancel out and you combine your elements together. So hydroiodic acid is just HI. And then also for c, +1, -1, the numbers are the same, so you just combine them together. So hydrofluoric acid is HF. For b, hydroselenic acid, we have here different numbers in the charges. Remember, when the numbers are different, they crisscross. So one from here comes here, 2 from here comes here, so that means, hydroselenic acid is H2Se. If you don't quite remember these rules, make sure you go back and take a look at our videos talking about writing ionic compounds. It's the same kind of principles behind this. We look at the ions, the charges, if the numbers are the same, they simply cancel out. If the numbers are different, then they crisscross. Using this helped us to determine each one of these 3 binary acids. Now that we've done this example, move on to the next video where we take a look at a practice problem.
Give the systematic name for the following compound:H2S
Give the systematic name for the following compound:HCN
Naming Acids
Video transcript
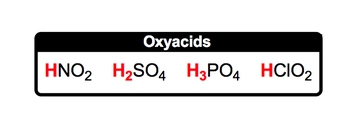
When it comes to oxyacids, oxyacids represent our second type of acid. Now we're going to say they represent covalent compounds because they're acids, they contain hydrogen ion as well, but now they're bonded to a polyatomic ion containing oxygen. And it's pretty clear why that is because the name is oxy, highlighting the fact that we have oxygen present now. So how could we form an oxyacid? Well, here we have our H+ ion, so H+, and let's just think of a polyatomic ion that possesses oxygen. Here, we could have NO3-. NO3- is our nitrate ion, and remember, when the numbers in the charges are the same, they just simply cancel out and you combine together your elements. So HNO3 would represent an oxyacid. It is covalent because it possesses only non-metals. It has your hydrogen ion at the beginning of the compound structure and altogether we have our oxyacid. Now that we know how they're formed and what they represent, click on to the next video and let's go over the rules for naming them.
Oxyacids contain a hydrogen ion connected to a polyatomic ion with oxygen.
Naming Acids
Video transcript
When it comes to naming oxyacids, it's first important to remember your polyatomic ions. Now if you don't remember your polyatomic ions or you haven't watched my videos on them, I suggest you pause this video and go back and take a look at those videos first. Then come back and we can tackle naming oxyacids together. Alright. So for those of you who are ready, let's go. We're going to say here rules for naming oxyacids. Rule 1, if the polyatomic ion ends with -ate, then change the ending when it's in its acid form to -ic acid. Here, we have a memory tool that will help us. So here we're gonna say I ate in acid, and it was icky. Now, don't go biting into acids or eating them in any way, but just remember that -ate goes with icky. Okay? So in its polyatomic ion form, it's -ate, but in its acid form, we change the ending to -ic acid. So here we have H+ with NO3-. Remember, NO3- is your nitrate ion. When I combine them together to give me HNO3, that is our oxyacid form. Here, the -ate ending changes to -ic, acid. So nitrate becomes nitric acid. But remember, we also have polyatomic ions that end with -ite. What do we do in those situations? Well, we're gonna say if the polyatomic ion ends with -ite, then in its acid form, we'll change the ending to -ous acid. And again, we have a great memory tool, so I only bite into things that are delicious. So this will help us remember, if we have a polyatomic ion that ends with -ite, in its acid form it becomes -ous ending, -ous acid. So here we have H+ with NO2-. NO2- is our nitrite ion. When it combines with H+, we get HNO2, which is our oxyacid form. The -ite ending changes to -ous acid. So nitrite becomes nitrous acid. So again, unless you know your polyatomic ions, it gets pretty tricky in terms of naming the oxyacid. Now that we've seen the rules for this, let's continue on with some questions and test what we've learned.
Naming Acids Example 2
Video transcript
In this example question, it says to write the formula for each of the following compounds. Alright. So for a, we have H₂ CO₃. Think about what is making up this oxyacid. Well, we know that this is H+, and we know here this CO₃ is altogether; the 2 that comes here originated from the CO₃ because here, this is a carbonate ion. Remember, the ending is "ate". Remember, I ate an acid, and it was icky. So that means that "ate" will become "ic acid". So that means that this represents carbonic acid. So H₂ CO₃ is carbonic acid.
Next, H₃ PO₃. We know we have H+ here, and we know that PO₃ here, in terms of a polyatomic ion, was 3-. We also know based on what we've seen in the polyatomic ion videos, that this would be called phosphite. So remember, if it ends with "ite", we're going to say here that I bite into things that are delicious. Right? "Ite" becomes "ous acid". Also, remember that phosphorus, we use more than just the base name. We also include "or," and then it would be "ous acid". So this is phosphorous acid.
Finally, we have H₂ SO₄, which is composed of H+ and SO₄, which is a polyatomic ion that is 2-. It's one of our common types of tetraoxides. Alright. So this is called sulfate. It ends with "ate", so that means that our acid form would be "ic acid". Just like phosphorus, sulfur uses more than just the base name. So we actually use the whole name sulfur, and remember, it becomes "ic acid". So this is sulfuric acid as the name for our oxyacid. So, again, you have to remember your polyatomic ions because they go hand in hand with learning and recognizing the oxyacid forms that exist. Now that we've done this example, let's continue onward with more practice on naming oxyacids.
Write the formula for the following compound:Hypobromous acid
Write the formula for the following compound:Cyanic acid
Here’s what students ask on this topic:
What are the rules for naming binary acids?
Naming binary acids involves three main steps:
1. **Prefix**: Use the prefix "hydro-" to indicate the presence of hydrogen.
2. **Base Name**: Use the base name of the nonmetal element. For most nonmetals, this is the root of their name. However, for sulfur and phosphorus, use the full name (sulfur) or a modified form (phosphor-).
3. **Suffix**: Add the suffix "-ic acid" to the base name.
For example, HCl is named hydrochloric acid, and H2S is named hydrosulfuric acid.
 Created using AI
Created using AIHow do you name oxyacids?
Naming oxyacids depends on the ending of the polyatomic ion:
1. **If the polyatomic ion ends in "-ate"**: Change the ending to "-ic acid". For example, HNO3 (nitrate) becomes nitric acid.
2. **If the polyatomic ion ends in "-ite"**: Change the ending to "-ous acid". For example, HNO2 (nitrite) becomes nitrous acid.
Remembering these rules is crucial for correctly naming oxyacids.
 Created using AI
Created using AIWhat is the difference between binary acids and oxyacids?
Binary acids consist of hydrogen and a nonmetal anion, and they do not contain oxygen. Examples include HCl (hydrochloric acid) and H2S (hydrosulfuric acid).
Oxyacids, on the other hand, contain hydrogen bonded to a polyatomic ion that includes oxygen. Examples include HNO3 (nitric acid) and H2SO4 (sulfuric acid).
The naming conventions for these acids also differ, with binary acids using the "hydro-" prefix and "-ic acid" suffix, while oxyacids change the polyatomic ion endings from "-ate" to "-ic acid" and "-ite" to "-ous acid".
 Created using AI
Created using AIWhy is acetic acid an exception to the usual acid naming rules?
Acetic acid (CH3COOH) is an exception because it does not follow the typical structure of acids starting with a hydrogen ion. Instead, the hydrogen ion is at the end of the formula. Despite this, it is still considered an acid because it can donate a hydrogen ion (H+) in solution. This exception highlights the importance of understanding the underlying chemistry rather than relying solely on naming conventions.
 Created using AI
Created using AIHow do you determine the name of an acid from its chemical formula?
To determine the name of an acid from its chemical formula, follow these steps:
1. **Identify if it is a binary acid or an oxyacid**:
- **Binary acids**: Consist of hydrogen and a nonmetal. Use the prefix "hydro-", the base name of the nonmetal, and the suffix "-ic acid". For example, HCl is hydrochloric acid.
- **Oxyacids**: Contain hydrogen and a polyatomic ion with oxygen. If the polyatomic ion ends in "-ate", change it to "-ic acid". If it ends in "-ite", change it to "-ous acid". For example, H2SO4 (sulfate) becomes sulfuric acid, and H2SO3 (sulfite) becomes sulfurous acid.
 Created using AI
Created using AI