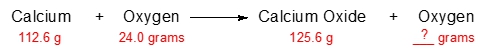In 1789, the French chemist, Antoine Lavoisier, who's credited as being another father of chemistry, originated the law of conservation of mass. Now, the law states that in a chemical reaction, no matter is created or destroyed, but instead changes form. In a chemical reaction, we're going to say that the compounds before the arrow, so in this case H2 and O2, would be our reactants, and those after the arrow, in this case, H2O, would be our products. According to Lavoisier, all other reactants are converted to product with nothing lost. So to think about it, we could say that originally at the beginning of the reaction, we have H2 and O2, and we're mixing them together. Their combined amount of both of them together, let's say it's a 100 grams. Well, according to the law of conservation of mass, nothing is truly lost. It's just converted from one form to another. So all 100 grams of my reactants should be converted entirely into the product. So at the end, I'd have exactly 100 grams of the product. All of this would be gone. All of this has been transformed into H2O. So what this tells me is that if you know the total mass of your reactants in the beginning, then they should equal the total mass of products that you create at the end. So this is an incredibly important idea when it comes to the law of conservation of mass. That'll connect to other ideas later on such as stoichiometry and solution chemistry. So just remember, when it comes to the law of conservation of mass, it helps us to determine eventually the amount of product we could potentially form.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Law of Conservation of Mass - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AI"In a chemical reaction, no matter is created or destroyed, but instead changes form."
Lavoisier's Conservation of Mass
Law of Conservation of Mass
Video transcript
According to Lavoisier, all reactants are completely converted into products.
Law of Conservation of Mass Example 1
Video transcript
In this example question, it asks how many grams of water vapor will form if 25 grams of hydrogen gas mixes with 12 grams of oxygen gas? Now, applying what we just learned about the law of conservation of mass, this isn't going to be too difficult. Because remember, it states that the mass, the total mass of reactants, will equal the total mass of products. Here we have the masses of the reactants which are 25 and 12, so when we add them together, that'll give us the total mass of the product that could potentially form. So the answer here would be 37 grams of the product. Now, if we want to follow the law and the rules for significant figures, technically, this has 3 significant figures, and this has 3 significant figures, so the better answer would be 37.0 grams of water vapor, which is just water as a gas. So just remember, when we're simply applying the law of conservation of mass, the amount of reactants equals the amount of products. Now, later on, when we go into more advanced forms of calculations with chemical reactions, we're going to see that it's not always true. In those cases, we'll learn new mechanics and new approaches to answer those particular types of questions.
Following the Law of Conservation of Mass, predict the minimum amount of nitrogen that will react with 50.0 grams of hydrogen to produce 92.5 grams of ammonia.
Nitrogen + Hydrogen → Ammonia
Predict the amount of oxygen gas that will remain after the reaction of 112.6 grams of calcium with 24.0 grams of oxygen.
Here’s what students ask on this topic:
What is the law of conservation of mass and who discovered it?
The law of conservation of mass states that in a chemical reaction, matter is neither created nor destroyed but is transformed from one form to another. This means that the total mass of the reactants equals the total mass of the products. This fundamental principle was discovered by the French chemist Antoine Lavoisier in 1789. Lavoisier's work laid the foundation for modern chemistry by emphasizing the importance of careful measurement and the concept that mass remains constant in a closed system during chemical reactions.
 Created using AI
Created using AIHow does the law of conservation of mass apply to chemical reactions?
The law of conservation of mass applies to chemical reactions by ensuring that the total mass of the reactants equals the total mass of the products. For example, when hydrogen (H2) and oxygen (O2) react to form water (H2O), the combined mass of H2 and O2 before the reaction will equal the mass of H2O produced. This principle is crucial for balancing chemical equations, as it ensures that the number of atoms for each element is the same on both sides of the equation, reflecting the conservation of mass.
 Created using AI
Created using AIWhy is the law of conservation of mass important in stoichiometry?
The law of conservation of mass is important in stoichiometry because it allows chemists to predict the amounts of products formed in a chemical reaction based on the initial amounts of reactants. By knowing that mass is conserved, chemists can use balanced chemical equations to calculate the theoretical yield of a reaction. This involves using molar ratios derived from the balanced equation to determine how much product can be formed from given quantities of reactants, ensuring that no mass is lost or gained in the process.
 Created using AI
Created using AICan you provide an example of the law of conservation of mass in a chemical reaction?
Sure! Consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O). The balanced chemical equation is:
If we start with 4 grams of H2 and 32 grams of O2, the total mass of reactants is 36 grams. According to the law of conservation of mass, the mass of the water produced will also be 36 grams. This demonstrates that the total mass remains constant, with matter simply changing form from reactants to products.
 Created using AI
Created using AIHow does the law of conservation of mass relate to balanced chemical equations?
The law of conservation of mass is directly related to balanced chemical equations. A balanced chemical equation ensures that the number of atoms for each element is the same on both sides of the equation, reflecting the conservation of mass. For example, in the reaction:
There are 4 hydrogen atoms and 2 oxygen atoms on both sides of the equation. This balance ensures that the mass of the reactants equals the mass of the products, adhering to the law of conservation of mass.
 Created using AI
Created using AI