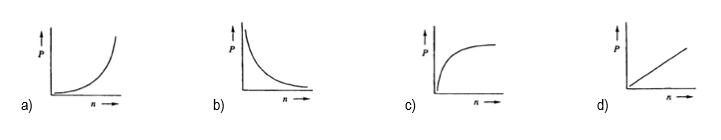
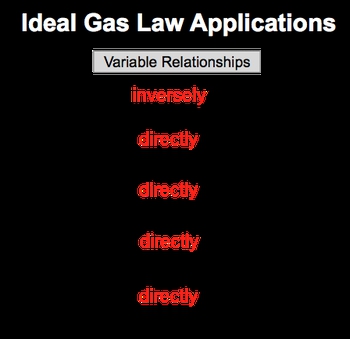
By rearranging the ideal gas law, we can establish direct and inverse relationships between its variables. So here we have the ideal gas law formula, which is PV=nRT. R is our gas constant. Because it's constant, we don't have to relate it to the other variables. Next, we have our variable chart and we'll see what effect happens to the other variables if pressure is increasing and volume is increasing. And then in our variable relationships, we'll take a look at different pairs of variables and see if they are directly proportional or inversely proportional to one another. Alright. So first, let's look at what happens when I increase pressure. Let's see what is the effect that it has on volume. Our equation is PV=nRT. We're not gonna talk about nRT because we're looking strictly at the relationship between pressure and volume. Now to make them relate to one another, we're gonna say that this is equal to a constant, let's just say that it's 1, and we're gonna bring volume over to the other side. By doing that, we can see that the relationship is that pressure is equal to 1V. Pressure here is a numerator, volume is a denominator. If you've watched my other videos in terms of direct and inverse relationships, realize that because they're on different levels, they're inversely proportional. If volume is increasing and the top is staying constant, if volume is increasing that means pressure would be decreasing. If volume is decreasing, pressure would be increasing. So coming back to our variables chart we're gonna say that the relationship is if we increase my pressure that's gonna decrease my volume because they have an inversely proportional relationship or opposite relationship. Now let's compare pressure and moles. Okay. So pressure and moles, ignore volume R and T. So then this would just be pressure equals moles. They're both numerators, both on the same level. If I increase 1 that's gonna cause an increase in the other. So increasing pressure while keeping all the other variables out increases my moles. Since they're increasing or decreasing together, they are directly proportional. Next, pressure equals temperature. Right? Ignore volume, moles, and R because we're focusing only on pressure and temperature. Again, they're both numerators so they both increase together. So they are directly proportional. Alright. Now that we've done that let's look at volume. So volume, we're increasing volume and we're trying to see what effect it has on my moles and my temperature here. So focus on only moles and volume. Ignore pressure, ignore R and T. Volume and moles are on the same level with one another. They're both numerators, so increasing one would cause an increase in the other. Why? Because they are directly proportional. And then finally, volume equals temperature. So at this point you've seen us do the other ones. Give yourself a second pause the video if you want and see what the relationship between volume and temperature would be. Alright. Hopefully, you've done that and realize that volume and temperature, both of them considered numerators, both on the same level, an increase in one will cause an increase in the other. Why? Because they too would be directly proportional. So these are the relationships that we can establish between the variables of the ideal gas law. And we can see that really only pressure and volume is where we would see an inverse relationship when doing these pairings of the variables. So keep this in mind if you're faced with any type of theoretical question where they're increasing or decreasing one variable, and asking for the effect on another within the ideal gas law.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
The Ideal Gas Law Applications - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIThe Ideal Gas Law Applications establish both the direct and inverse relationships between the Ideal Gas Law variables.
Understanding the Ideal Gas Law Applications
The Ideal Gas Law Applications
Video transcript
The Ideal Gas Law Applications Example 1
Video transcript
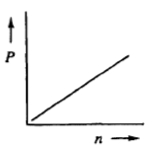
If the number of moles n inside a container were tripled while keeping the pressure p constant, what will happen to the volume v? Alright. So in this question, they're telling us that our pressure is being held constant, so just ignore it. What's changing are our moles and we have to understand what effect that will have on my volume. Looking at the ideal gas law, pv=nrt, we're only paying attention to volume and moles. The other variables we can remove. By doing that we see that it simplifies further into v=n. So this means that volume and moles have a direct relationship to each other; they're directly proportional. Meaning, whatever happens to one, the same thing happens to the other. Since our moles are being tripled, that would mean that my volume would also have to be tripled. This means that the answer would have to be option C. So again, sometimes you'll be faced with questions like this where you're not given actual numbers, but you have to understand the direct or possibly inverse relationship between a pair of variables from the ideal gas law.
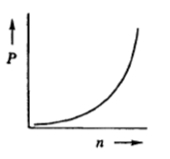
The relationship between the partial pressure of a gas (P) and the number of moles of that gas (n) is best represented by which of the following graphs?
Here’s what students ask on this topic:
What is the ideal gas law and how is it used in chemistry?
The ideal gas law is a fundamental equation in chemistry that describes the behavior of ideal gases. It is expressed as , where is pressure, is volume, is the number of moles, is the gas constant, and is temperature. This law helps predict how gases will respond to changes in pressure, volume, temperature, and the number of moles. It is crucial for solving problems related to gas behavior and thermodynamics in chemistry.
 Created using AI
Created using AIHow are pressure and volume related in the ideal gas law?
In the ideal gas law, pressure and volume are inversely proportional to each other. This means that if the pressure of a gas increases, its volume decreases, and vice versa, provided the temperature and the number of moles remain constant. Mathematically, this relationship is expressed as , where is a constant. This inverse relationship is crucial for understanding how gases behave under different conditions.
 Created using AI
Created using AIWhat is the relationship between pressure and temperature in the ideal gas law?
In the ideal gas law, pressure and temperature are directly proportional to each other when volume and the number of moles are held constant. This means that if the temperature of a gas increases, its pressure also increases, and vice versa. Mathematically, this relationship can be expressed as , where is a constant. This direct relationship is essential for understanding how gases respond to temperature changes.
 Created using AI
Created using AIHow does the ideal gas law explain the relationship between volume and temperature?
According to the ideal gas law, volume and temperature are directly proportional to each other when pressure and the number of moles are constant. This means that if the temperature of a gas increases, its volume also increases, and vice versa. This relationship is expressed mathematically as , where is a constant. Understanding this direct relationship is crucial for predicting how gases will behave under different thermal conditions.
 Created using AI
Created using AIWhat is the significance of the gas constant (R) in the ideal gas law?
The gas constant, denoted as , is a crucial component of the ideal gas law. It provides the necessary proportionality to relate pressure, volume, temperature, and the number of moles of a gas. The value of depends on the units used for pressure, volume, and temperature. Commonly, is 8.314 J/(mol·K) or 0.0821 L·atm/(mol·K). The gas constant ensures that the ideal gas law can be applied universally to different gases under various conditions.
 Created using AI
Created using AI