Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolytes
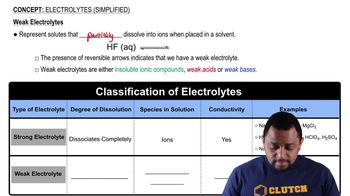
Electrolytes are substances that dissociate into ions when dissolved in water, allowing the solution to conduct electricity. They are classified into strong electrolytes, which completely dissociate into ions, weak electrolytes, which partially dissociate, and nonelectrolytes, which do not dissociate at all. Understanding the behavior of electrolytes is crucial for analyzing chemical reactions in aqueous solutions.
Recommended video:
Electrolytes (Simplified) Example 3
Strong vs. Weak Electrolytes
Strong electrolytes, such as sodium chloride, fully ionize in solution, resulting in a high concentration of ions. In contrast, weak electrolytes, like ammonia (NH₃), only partially ionize, leading to a mixture of un-ionized molecules and ions in solution. This distinction is important for predicting the conductivity and reactivity of solutions in chemical processes.
Recommended video:
Electrolytes (Simplified) Concept 2
Ammonia as a Weak Electrolyte
Ammonia (NH₃) is classified as a weak electrolyte because it does not fully dissociate in water. Instead, it establishes an equilibrium with water, forming ammonium ions (NH₄⁺) and hydroxide ions (OH⁻). This partial ionization is characteristic of weak electrolytes, which influences the pH and conductivity of the solution.
Recommended video:
Electrolytes (Simplified) Concept 2
 Verified step by step guidance
Verified step by step guidance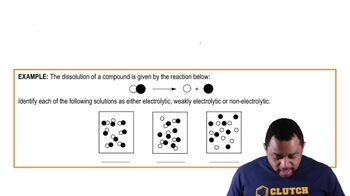


 2:38m
2:38m