Refer to the cellobiose structure in Worked Example 20.5. How would you classify the link between the monosaccharides in cellobiose?
Draw the structure of the α and ß anomers that result from the reaction of methanol and ribose. Are these compounds acetals or hemiacetals?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Anomers

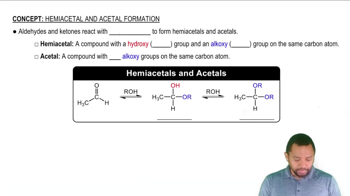
Acetals and Hemiacetals
Cyclization of Sugars

In the monosaccharide hemiacetal shown below number all the carbon atoms, identify the anomeric carbon atom, and identify it as the α or ß anomer. <IMAGE>
l-Fucose is one of the naturally occurring l monosaccharides. It is present in the short chains of monosaccharides by which blood groups are classified (see the Chemistry in Action “Cell-Surface Carbohydrates and Blood Type” on p. 640). Compare the structure of l-fucose shown in the margin with the structures of α- and ß-d-galactose and answer the following questions.
d. Fucose” is a common name. Is 6-deoxy-l-galactose a correct name for fucose? Why or why not? <IMAGE>
Draw the structures of an aldopentose and a ketohexose.
Draw the structures of a ketohexose.
Identify the following as diastereomers, enantiomers, and/or anomers. (a) ß-d-fructose and
ß-d-fructose (b) d-galactose and l-galactose (c) l-allose and d-glucose (both aldohexoses)
