Why do allosteric enzymes have two types of binding sites?
How can you distinguish between a competitive inhibitor and an uncompetitive inhibitor experimentally?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Competitive Inhibition
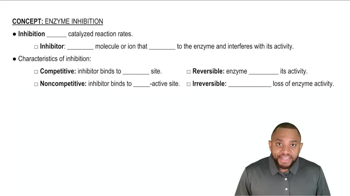
Uncompetitive Inhibition

Enzyme Kinetics

Identify and describe the chemical change in the lyase-catalyzed reaction in Table 19.4 that involves fumarate and malate. Identify the substrate(s) and product(s). <IMAGE>
Which of the following reactions can be catalyzed by a decarboxylase?
a. <IMAGE>
b. <IMAGE>
How will changing the conditions in an enzymatic reaction affect the rate of that reaction? Explain why in each case.
f. Decreasing the amount of substrate by half
Why are irreversible enzyme inhibitors referred to as poisons?
Apple slices and peeled potatoes rapidly brown in open air due to the presence of phenolases. Phenolases cause the oxidation of phenolic molecules like tyrosine to quinones, colored molecules responsible for the brown colors seen. An experiment comparing the time it took for a change to occur in the color of apple slices versus potato slices was done to test for phenolase activity. Then, a second experiment was done with new apple and potato slices with H2O2 measuring time until bubbles appeared. <IMAGE>
b. Which sample contains more catalase? Why?
