Consider the following unnatural amino acid: <IMAGE>
c. Draw the cyclic ester resulting from the intramolecular reaction of the hydroxyl group of this amino acid with its carboxyl group (cyclic esters are called lactones).
 Verified step by step guidance
Verified step by step guidance

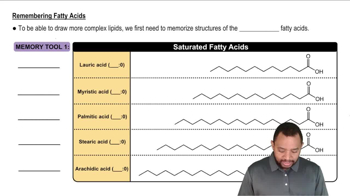

Consider the following unnatural amino acid: <IMAGE>
c. Draw the cyclic ester resulting from the intramolecular reaction of the hydroxyl group of this amino acid with its carboxyl group (cyclic esters are called lactones).
Draw structures of the carboxylic acids and alcohols you would use to prepare each ester in Problem 17.54.
Household soap is a mixture of the sodium or potassium salts of long-chain carboxylic acids that arise from saponification of animal fat.
b. Draw the structures of the soap molecules produced in the following reaction: <IMAGE>
Each of the following materials has an ester that is responsible for its smell and/or flavor. Search the internet and determine what that ester is, draw its structure, and what carboxylic acid and alcohol are used to form it.
a. Juicy Fruit gum flavoring
Based on the structure shown for retinol (vitamin A) and the names of the two related forms of vitamin A, retinal and retinoic acid, what do you expect to be the structural differences among these three compounds?
Draw structures of the amides that can be made from the following reactants:
a. CH₃NH₂ + (CH₃)₂CHCOOH →?