Textbook Question
Give the structure of the repeating units in the polymers that are formed in the reactions of the following compounds.
a. <IMAGE>
140
views
 Verified step by step guidance
Verified step by step guidance
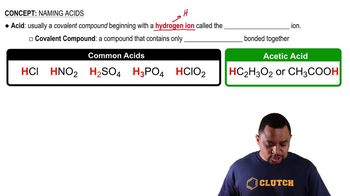


Give the structure of the repeating units in the polymers that are formed in the reactions of the following compounds.
a. <IMAGE>
N-Acetylglucosamine (also known as NAG) is an important component on the surfaces of cells.
b. Draw the structures of the products of acid hydrolysis.
Write the equation for the ionization of hexanoic acid in water at pH 7.4. (Hint: See Section 17.2.)
Suppose you have a sample of benzoic acid dissolved in water.
c. Finally, assume that aqueous HCl is added to the solution from (b) until pH 2 is reached. Draw the structure of the major organic species present.
Give systematic names for the following carboxylic acids:
d. <IMAGE>
Give systematic names for the following carboxylic acids:
d. CH₃(CH₂)₅COOH