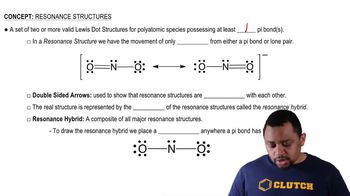
Write structural formulas for compounds that meet the following descriptions:
b. An alkene with a chemical formula of C10H12, that has cis–trans isomers and contains a benzene ring.
 Verified step by step guidance
Verified step by step guidance

Write structural formulas for compounds that meet the following descriptions:
b. An alkene with a chemical formula of C10H12, that has cis–trans isomers and contains a benzene ring.
Draw structures corresponding to the following IUPAC names:
a. trans-2-Pentene
b. trans-3,4-Dimethyl-3-hexene
c. 2-Methyl-1,3-butadiene
d. trans-3-Heptene
Which of the following substances exist as can cis–trans isomers? Draw both isomers for those that do.
a. 2,3-Dimethyl-2-pentene (condensed structures only)
b. 2-Methyl-2-hexene (both condensed and line structures)
c. 2-Hexene (line structures only)
If 2-methyl-2-pentene were converted into 1-hexene, what kind of reaction would that be?
Identify the type of reaction for the following:
a. <IMAGE>
b. <IMAGE>
What alkene could you use to make the following products? Draw the structure of the alkene, and tell what other reagent is also required for the reaction to occur.
a. <IMAGE>