Here are the essential concepts you must grasp in order to answer the question correctly.
De-icing Compounds
De-icing compounds are substances applied to surfaces like roads and sidewalks to lower the freezing point of water, thereby preventing ice formation. Common examples include salt (sodium chloride) and calcium chloride, which work by disrupting the formation of ice crystals, making it easier to clear surfaces during winter conditions.
Recommended video:
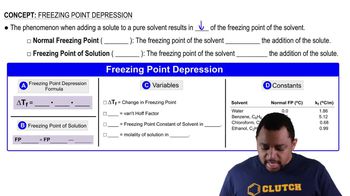
Freezing Point Depression
Freezing point depression is a colligative property that describes how the addition of a solute (like salt) to a solvent (like water) lowers the temperature at which the solvent freezes. This principle is crucial for understanding how de-icing agents function, as they allow for the melting of ice at temperatures where pure water would remain frozen.
Recommended video:
Freezing Point Depression Concept 1
Indicators in De-icing Compounds
Indicators are colored compounds added to de-icing agents to provide visual cues about their presence and effectiveness. These dyes help municipalities and homeowners see where the de-icing material has been applied, ensuring even coverage and preventing over-application, which can be harmful to the environment.
Recommended video: