Here are the essential concepts you must grasp in order to answer the question correctly.
Concentration (m/v)
Mass/volume (m/v) concentration expresses the mass of solute per 100 mL of solution. A 0.50% (m/v) solution means there are 0.50 grams of solute (boric acid) in every 100 mL of solution. This concept is crucial for understanding how to prepare solutions with specific concentrations.
Recommended video:
Percent Concentrations Concept 1
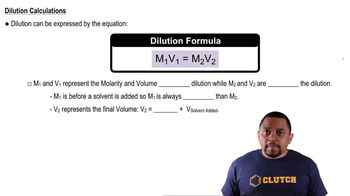
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. In this case, to prepare a 0.50% (m/v) solution from a more concentrated stock solution, one must calculate the appropriate volume of stock solution and the amount of water needed to achieve the desired concentration.
Recommended video:
Volume Measurement
Accurate volume measurement is essential in preparing solutions. For this task, one must measure 500.0 mL of the final solution using appropriate lab equipment, such as a graduated cylinder or volumetric flask, to ensure the correct concentration of boric acid is achieved.
Recommended video:
Measuring Radioactivity Concept 1
 Verified step by step guidance
Verified step by step guidance