Now, coenzymes are important in metabolic reactions. We're going to say the driving force of catabolism is the oxidation of molecules in order to release energy. We're going to say this is accomplished by coenzyme cycling between their oxidized and reduced forms. Now, here we're going to say the reduced forms act as electron carriers that carry energy that is ultimately passed to the bonds of ATP. If we take a look here, we're going to say that we have substrate A. And to show its reduced form and remember, reduction here is in terms of having hydrogen or not, gaining hydrogen or not. So this substrate A would be the reduced form of it. To show that, we just show a hydrogen on it. Here it undergoes oxidation where it will lose its hydrogen. So if this thing here is losing its hydrogen, where is it going? Well, if it's losing its H, it's handing it over to the coenzyme. Here, the coenzyme in its oxidized form doesn't have it, but it gets reduced in this process and now it's in its reduced form. The coenzyme is the carrier of the electrons in the form of this hydrogen component. That hydrogen is not only H, it's the electrons in the bond with it. That reduced form can then take itself, become oxidized back to its oxidized form. When it becomes oxidized, it's basically handing over its H to substrate B. Substrate B gains that H, and now we have it in its reduced form here. So, basically, these coenzymes are kind of acting as a carrier. They're taking electrons from one molecule and handing them over to another one later on. This, again, ultimately is to help us to create lots and lots of ATP at the end. So as we progress through our topics, we'll see how this exactly is done.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Coenzymes in Metabolism: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AICoenzymes play a crucial role in metabolic reactions, particularly in catabolism, where they facilitate the oxidation of molecules to release energy. Key coenzymes include NAD+, which reduces to NADH by accepting electrons and hydrogen, and FAD, which reduces to FADH2 through the addition of electrons and hydrogen. Coenzyme A carries acetyl groups to the Krebs Cycle, essential for ATP production. Understanding these processes is vital for grasping energy metabolism and the biochemical pathways involved in cellular respiration.
Coenzymes in Metabolism Concept 1
Video transcript
Coenzymes in Metabolism Example 1
Video transcript
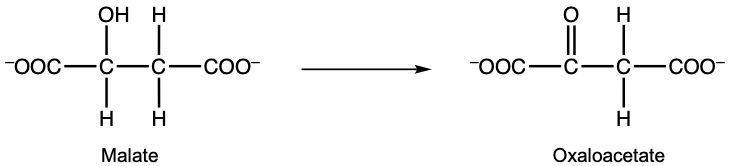
Here in this example, it says, consider the reaction given below and correctly identify the oxidizing agent. Remember, your oxidizing agent is what's been reduced. And what's been reduced has gained electrons or, in this case, Hydrogen with those electrons. And remember, when we're looking at oxidizing agent or reducing agent, those are the reactants. We look at the product side to see where the electrons went, but then we look back at the reactant to determine, okay, this was reduced, and this was oxidized. If we take a look here, we have malate as a reactant, and then over here, we have Oxaloacetate. Here we have NAD+ which is a coenzyme. But if we look, NAD+ in this form as a reactant, but then over here, it's gained a hydrogen. And not only a Hydrogen but electrons. That's why it's neutral now. So we're going to say NAD+ gained electrons and Hydrogen and so it was reduced, and therefore it's the oxidizing agent. Now this question doesn't ask this, but we could also say that Malate had an alcohol group here. This alcohol group was oxidized into a ketone portion here. Remember, we learned this several chapters ago that alcohols can be oxidized into carbonyl groups C=O. So this thing was oxidized. Therefore, it's the reducing agent. The question doesn't ask this, but we're just trying to cover everything to see the difference. But again, for this particular one, the oxidizing agent would be NAD+.
Nicotinamide Adenine Dinucleotide Concept 2
Video transcript
So, Nicotinamide Adenine Dinucleotide, or NAD. We're going to say here the Nicotinamide group of NAD+ is the site of reduction that is seeking to become neutral, so we want to lose that charge. If we look here at NAD+, it's oxidized form. Nitrogen, remember when it makes 4 bonds, is positively charged. We're trying to remove this positive charge here. We're going to say the reduction occurs by accepting 2 electrons. We have 2 electrons here. And, we're going to say here to gain 1 Hydrogen. So here, we're going to gain the hydrogen, the hydrogen will be gained right here. And, initially, we're going to start with 2 Hydrogens. We used 1 to attach it to our structure to create NADH, the reduced form, so it'd be one left.
Now, here are the results of the reduction of NAD+ to NADH. If we take a look here, the way this works is that both of these H+s have no electrons whatsoever. And we're going to say here that 2 of these electrons would have bound to 1 of these H+s. So 2 electrons plus the 1 H+ would give us an H-. Gain those 2 electrons. It's this H- that is attaching itself to this site right here. By attaching it to that site there, that's how it's connected to it. And attaching there causes a shifting of our pi bonds. That's why the structure now looks like this: Nitrogen here is no longer making 4 bonds so it's no longer positively charged. It has a lone pair on it though. Again, it's not important to see how these pi bonds move around through resonance. What's important to understand here is that we're going to have this being the site where the H- attaches. So it's important to know you have NAD+ plus 2 electrons plus 2 H+ giving us this structure. Being able to show the structure, and that's the reduced form. And then having 1 H+ left over as a product. Right? So that's what we should take away from this particular example reaction.
Nicotinamide Adenine Dinucleotide Example 2
Video transcript
Which of the following correctly identifies the molecules in the given reaction? So here we have lactate interacting with our coenzyme. Our enzyme is lactate dehydrogenase, and it's going to create pyruvate plus NADH plus H+. Now remember, NAD+ represents a coenzyme and it's in its oxidized form. If we look at our options, b would be out because NAD+ is not in its reduced form. Next, let's see. We can say that lactate dehydrogenase represents our enzyme. We can say here that this lactate represents our substrate. What kind of substrate what form is it in here? Well, here this is the oxidized form, and over here is its reduced form. So we'd say that NAD+ was reduced. Right? To give us this. Right now, it's in its oxidized form. If this is in its oxidized form and this is a redox reaction, the substrate would have to be in its reduced form. And we know it's in its reduced form because its OH group is later on oxidized to a carbonyl group. So the substrate here is in its reduced form. So, all of those are fine. Let's see. Then we're going to say, NAD+ is an oxidized coenzyme. Oxidized cofactor is just the umbrella term. Remember, cofactors can be either organic or inorganic. Pyruvate. What does pyruvate represent? Well, here this is the reduced substrate, so this would have to be the oxidized substrate. So this is good, this is good, and this is good. Now, it looks like option c or d could be an answer, but what's the best answer? Cofactor is just an umbrella term. We got to be more specific. NAD+ represents an organic cofactor. Therefore, it is a coenzyme. So option d would be the best answer here because it goes one step further in describing what NAD+ is. Yes. It's oxidized, but more specifically, it is an organic cofactor called a coenzyme. So option d would be our final answer.
Flavin Adenine Dinucleotide Concept 3
Video transcript
So FAD is called Flavin Adenine Dinucleotide. And we're going to say the flavin group of FAD is the site of reduction that has 2 Hydrogen atoms added to its Nitrogen atoms. Now, here we're going to say the reduction occurs by adding 2 electrons to 2 H+ in order to form 2 new covalent bonds. The result of this is the reduction of FAD to FADH2. So here we have our oxidized form of the flavin portion of FAD. We're reacting 2 electrons and 2 H+ here. And when we reduce this FAD, we get FADH2. The 2 Hydrogens that we gain, 1 is added here and 1 is added here. Notice that there is a shifting of our pi bonds in order to do this. What's most important is to understand that reduction is happening at this nitrogen and this nitrogen and we have the transformation of FAD to FADH2.
Flavin Adenine Dinucleotide Example 3
Video transcript
Here with this example, it states when FAD oxidizes the substrate, it converts the carbon single carbon bonds to carbon double bonded carbon bonds. Complete the following reaction. Alright. So here we have succinate. We have these 2 CH2 carbons in the middle, and then we have these carboxyl groups at the end. We're basically going to use FAD to go from these single bonded carbon carbon bonds to double bonded carbons. So, now those 2 carbons are double bonded. 4 bonds. This Carbon here we see making 3, so it would only have 1 Hydrogen on it. And then the same thing with this one. Here, I just chose to put them on opposite sides of each other. Doing this would go from succinate to what we call fumarate. Now, it's not important that we know what the name of this molecule is for this question. I'm just naming it for you. Now, where did those 2 Hydrogens go? Well, we see that Succinate lost Hydrogens, so it was oxidized, which would mean that FAD had to be reduced. And we know that when we reduce FAD, it becomes FADH2. So these will be our 2 products formed within this given reaction.
Coenzyme A Concept 4
Video transcript
Now, here we're going to say that coenzyme A is a coenzyme of synthase and it has a high energy thiol bond, so s h bond. It carries an Acetyl group to the Krebs Cycle for energy production by oxidation. If we take a look here, this whole structure represents Coenzyme A. Coenzyme A is made up of 3 portions. We have our ADP portion right here, which is Adenosine Diphosphate. Then we have here pantothenic acid. Pantothenic acid, another common name for it is vitamin B5. So that would be this portion right here. And then, finally, we have our Aminoethanethiol portion, which is this nitrogen portion with these 2 CH2s and then the SH at the end. Again, it's the SH group that has a high energy bond. That hydrogen can be removed from this structure. So just remember, Coenzyme A is just yet another simple type of coenzyme that exists.
Coenzyme A Example 4
Video transcript
Now, which of the following statements correctly describes Coenzyme A? Here we have to select all that apply:
- First, when an Acetyl group is released from Acetyl S CoA, it produces here just our CoA with our thiol group. So, we are removing this Acetyl group here and we replace it with a hydrogen. This is true.
- Vitamin B is present in the active site of our Coenzyme A. No, that's not the active site; it's the Aminoethanethiol portion, the portion that has the SH group that has the high energy bond.
- The primary role of CoA is to oxidize fatty acids. No, that's not its primary role. Its primary role is to carry an Acetyl group to the Krebs Cycle for it to become oxidized and generate energy.
- Coenzyme A is composed of Pantothenic acid, which is vitamin B5, Aminoethanol, and ADP. Yes. These are the three major components of our Coenzyme A.
So here, the only two answers that are true are options A and D.
Select the correct statement.
When FAD is reduced, it gains 2 hydrogen ions and 2 electrons, forming FADH.
NAD+ represents an oxidized form of the coenzyme, and acts as an oxidizing agent.
FADH represents an oxidized form of the coenzyme, and acts as a reducing agent.
After CoA is oxidized, it forms Acetyl CoA.
Is the following reaction an oxidation or reduction? Which coenzyme would be carrying this out, NAD+ or NADH?
oxidation, NAD+
oxidation, NADH
reduction, NAD+
reduction, NADH
Do you want more practice?
Here’s what students ask on this topic:
What role do coenzymes play in metabolic reactions?
Coenzymes are essential in metabolic reactions, particularly in catabolism, where they facilitate the oxidation of molecules to release energy. They act as carriers of electrons and hydrogen atoms, cycling between oxidized and reduced forms. For example, NAD+ reduces to NADH by accepting electrons and hydrogen, while FAD reduces to FADH2 through a similar process. These reduced forms then transfer the electrons to other molecules, ultimately aiding in the production of ATP, the cell's energy currency. Understanding these processes is crucial for grasping energy metabolism and the biochemical pathways involved in cellular respiration.
 Created using AI
Created using AIHow does NAD+ get reduced to NADH?
NAD+ (Nicotinamide Adenine Dinucleotide) gets reduced to NADH by accepting two electrons and one hydrogen ion (H+). The reduction occurs at the nicotinamide group of NAD+, which seeks to neutralize its positive charge. The reaction can be summarized as follows: NAD+ + 2e- + 2H+ → NADH + H+. In this process, one hydrogen atom attaches to the nicotinamide ring, and the other is released as a free proton (H+). This reduced form, NADH, then acts as an electron carrier in various metabolic pathways, including cellular respiration.
 Created using AI
Created using AIWhat is the function of FAD in metabolism?
FAD (Flavin Adenine Dinucleotide) functions as an electron carrier in metabolism. It gets reduced to FADH2 by accepting two electrons and two hydrogen ions (H+). The reduction occurs at the flavin group, forming two new covalent bonds with the hydrogen atoms. The reaction can be summarized as follows: FAD + 2e- + 2H+ → FADH2. FADH2 then participates in the electron transport chain, where it donates electrons to help produce ATP, the primary energy currency of the cell. This process is vital for cellular respiration and energy production.
 Created using AI
Created using AIWhat is the role of Coenzyme A in the Krebs Cycle?
Coenzyme A (CoA) plays a crucial role in the Krebs Cycle by carrying acetyl groups. It forms a high-energy thiol bond (S-H bond) with the acetyl group, creating Acetyl-CoA. This acetyl group is then delivered to the Krebs Cycle, where it undergoes oxidation to produce energy. The structure of Coenzyme A includes an ADP portion, pantothenic acid (vitamin B5), and an aminoethanethiol group. The high-energy bond in CoA is essential for transferring the acetyl group efficiently, facilitating the production of ATP through the Krebs Cycle and subsequent metabolic pathways.
 Created using AI
Created using AIHow do coenzymes like NAD+ and FAD contribute to ATP production?
Coenzymes like NAD+ and FAD contribute to ATP production by acting as electron carriers in metabolic pathways. NAD+ reduces to NADH by accepting electrons and hydrogen, while FAD reduces to FADH2 through a similar process. These reduced forms then donate electrons to the electron transport chain in the mitochondria. As electrons move through the chain, they create a proton gradient that drives the synthesis of ATP via ATP synthase. This process, known as oxidative phosphorylation, is the primary method of ATP production in aerobic organisms.
 Created using AI
Created using AIYour GOB Chemistry tutor
- FAD is a coenzyme for dehydrogenation.a. When a molecule is dehydrogenated, is FAD oxidized or reduced?
- FAD is a coenzyme for dehydrogenation.b. Is FAD an oxidizing agent or a reducing agent?
- FAD is a coenzyme for dehydrogenation.d. What is the form of FAD after dehydrogenation?
- The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which...
- The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which...
- Identify one or more coenzymes with each of the following components: ...
- Give the abbreviation for each of the following coenzymes: ...
- What coenzyme picks up hydrogen when a carbon–carbon double bond is formed?