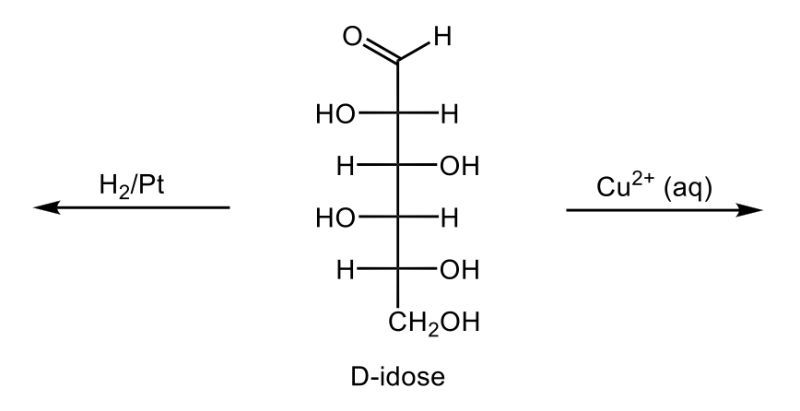
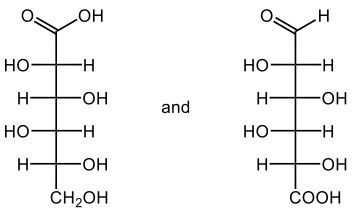
In this video, we're going to take a look at the oxidation of monosaccharides. Now recall that in Benedict's test, an aldehyde undergoes oxidation to a carboxylic acid and forms a brick-red precipitate. Here we're going to say that our aldose monosaccharides, so aldehyde sugars, produce what we call an aldonic acid. And aldonic acids are sugar acids, and again this happens upon oxidation. And the key takeaway from here is that we're changing the ending of our sugar name from 'ose' to 'onic acid'.
If we take a look here at this reaction we have D-ribose, which is a typical aldose sugar, so we have an aldehyde here. We're using copper(II) ion to oxidize this aldose sugar. We oxidize it into a carboxylic acid group, and now we're still be D, a D-sugar. It's still going to be ribe, and remember we're changing the 'ose' to 'onic acid', so it becomes ribonic acid. The brick-red precipitate happens in the form of copper(I) oxide here. So this is just a byproduct which would be our solid brick precipitate.
Now here, in addition to this, we have what's called a reducing sugar. This is a carbohydrate that produces a sugar acid upon oxidation, like D-ribose. And we're going to say here, Benedict's test can be used to detect a reducing sugar in solution. So, just remember in Benedict's test we're basically changing an aldose sugar into a carboxylic acid group, this transitions it away from an aldose sugar to an aldonic acid or sugar acid.