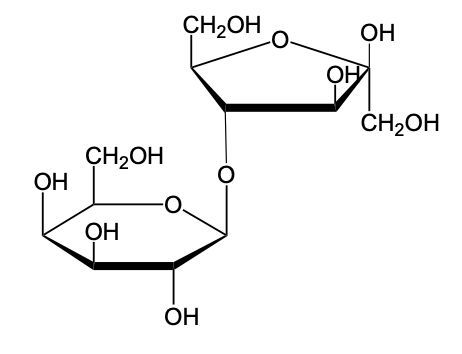
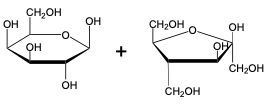
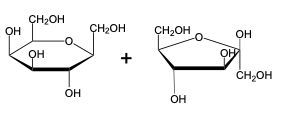
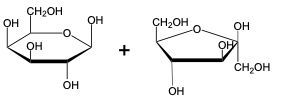
The glycosidic linkage is a crucial bond formed between monosaccharides, specifically involving the anomeric carbon of one sugar and a hydroxyl group of another. This process occurs through dehydration synthesis, which is the removal of a water molecule. The general equation for this reaction can be expressed as:
Monosaccharide 1 + Monosaccharide 2 → Disaccharide
In this context, a disaccharide is formed when two monosaccharides are linked together by a glycosidic bond. For example, when two molecules of Alpha-D-glucose interact, the anomeric carbon (carbon 1) of one glucose molecule reacts with the hydroxyl group (–OH) on carbon 4 of the other glucose molecule. This interaction leads to the loss of a water molecule, allowing the two sugars to bond.
As a result, the oxygen atom that was part of the hydroxyl group now forms a new bond with the anomeric carbon, creating the glycosidic linkage that connects the two monosaccharides. This transformation is essential for the formation of disaccharides, which play significant roles in biological systems, serving as energy sources and structural components.