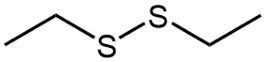
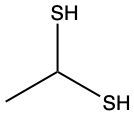
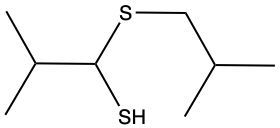
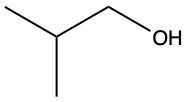
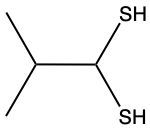
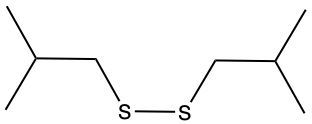
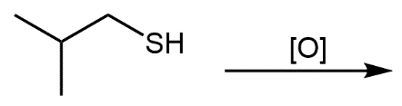Here we're going to say that the oxidation of a thiol with an oxidizing agent, for example, bromine water which is just Br2 in water, yields a disulfide. Now, the way it works is that we're going to have 2moles of thiol. They're going to undergo oxidation to help produce 1 mole of a disulfide. In this reaction, our SH bonds for each thiol molecule are broken and an SS bond is formed. So looking at it from an oxidation perspective, we're starting out with these 2 thiol molecules. Oxidation means the gaining of oxygen or the loss of hydrogen. So, in oxidation, we're going to lose these 2 hydrogens. The sulfurs themselves need to basically make up for the loss of a bond. So their best result is to bond with one another. Now we have these 2 sulfurs here bonded to each other. This becomes our disulfide product.
Now, going the opposite way, we could undergo reduction. And when we talk about reduction here, it means the addition of hydrogen. So in this way, we break the bond and give back hydrogens to each sulfur. Alright. So we're going to say here that oxidation shorthand is sometimes written as just O, and for reduction, we can write it in shorthand as just H. Oxidation means the gaining of oxygen or the loss of hydrogen. Reduction means the gaining of hydrogen. Alright. Now remember, reduction reactions, like we said, will convert the disulfide back into its thiol. So keep this in mind when we're talking about oxidation and reduction reactions, and how they connect thiols to disulfides.