This video, we're going to take a look at hydrogenation reactions. Under this reaction, 2 hydrogens are added to 1 pi bond. Now, due to the stability of H2, a catalyst is required to break the hydrogen-hydrogen bond first. So if we take a look here at this alkene, we have here our alkene, we only have 1 pi bond within our alkene, so we only require 1 mole of H2. Our catalyst being used here is usually some type of metal catalyst, we don't need to go too in-depth in terms of it, we'll save that for organic 1 and organic 2. But in essence what happens is we break the Pi bond in order to add the 2 hydrogens, one to each formally double-bonded carbon. At the end, we make an alkene product.
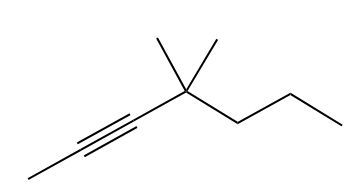
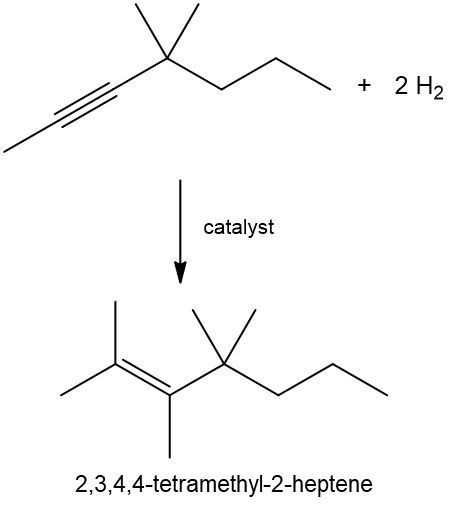
Now, with an alkyne we have 2 pi bonds now, and for every pi bond we require 1 mole of our reagent. Since there's 2 pi bonds, we need 2 moles of H2. We'd still use our catalyst, so here we add 4 hydrogen atoms, and look, whether we start with an alkene or an alkyne, the end product for a hydrogenation reaction is an alkane. So you're always trying to get to an alkane where you have only single-bonded carbon and hydrogen bonds. Right? So here we'd have 2 alkane products at the end.