- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Identify the substance that can exhibit cis-trans isomerism. If the substance exhibits isomerism, draw both isomers.
a. 2-methyl-2-pentene (both condensed and line structures)
b. 3-hexene (line structures only)
c. 2,3-dimethyl-2-hexene (condensed structures only)
Using condensed structural formulas, show all possible haloalkane isomers that satisfy the following descriptions: a total of five carbons, contain chlorine, and have the longest chain of four carbon atoms.
Which of the following statements correctly describe the characteristics of a compound's conformational isomers and structural isomers? Select all that apply.
I. Conformational isomers differ in the connectivity of their atoms.
II. Structural isomers have the same molecular formula but differ in the spatial arrangement of atoms.
III. Conformational isomers can be interconverted by simple rotations around single bonds.
IV. Structural isomers involve different bonding patterns between atoms.
V. Conformational isomers differ in their 3D spatial arrangement due to rotation around single bonds.
For the following alkene, determine whether it can exhibit cis–trans stereoisomerism. If it can, draw the correct cis and trans forms:
2-bromo-2-butene
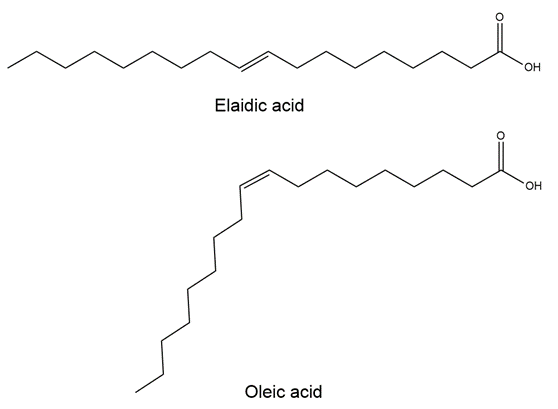
Consider the stereoisomers elaidic acid and oleic acid. Identify which is the cis-isomer and which is the trans-isomer.
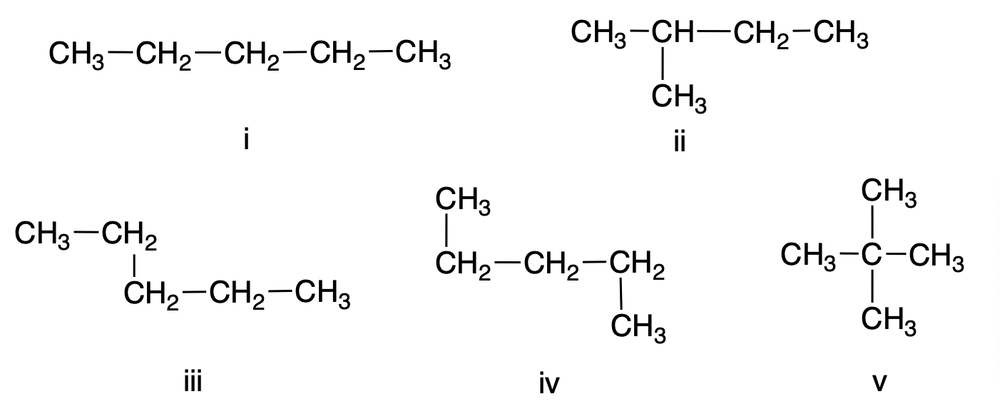
Which of the following condensed structural formulas does not represent an n-pentane conformer?
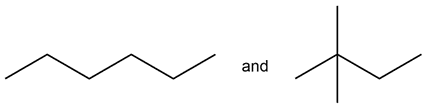
For the pair of molecules below, determine whether they are structural isomers, geometric (cis—trans) isomers, enantiomers, or identical molecules.