- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Determine if the given Fischer projections are identical or enantiomers.
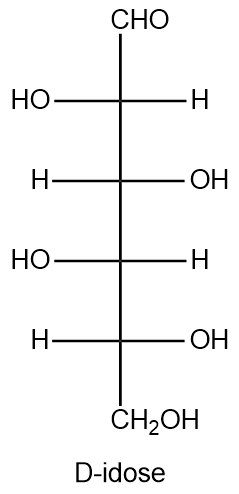
Using the Fischer projection of D-idose provided below as a reference, draw the structure of L-idose.
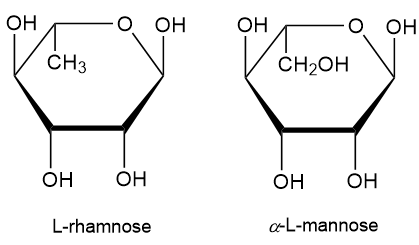
L-Rhamnose is a deoxy sugar that can be found in various plants. Compare the structure of L-rhamnose with the structure of α-L-mannose and answer the following question. Is 6-deoxy-L-mannose an appropriate name for L-rhamnose? Explain why or why not.
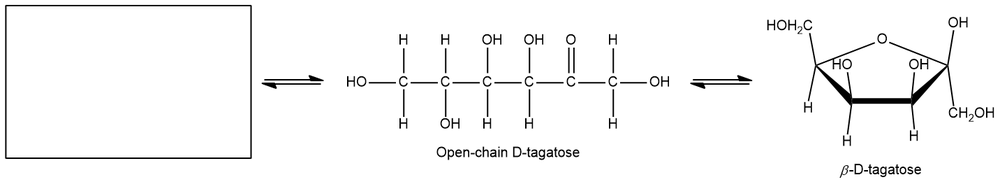
Provide the missing structure in the mutarotation rotation reaction of D-tagatose given below.
Volemitol is a naturally occurring seven-carbon sugar alcohol found in plants, red algae, fungi, mosses, and lichens. Volemitol is formed when D-manno-heptose undergoes reduction at carbon 1. Based on this information, draw the structure of volemitol.
D-mannitol is a sugar alcohol used as a sweetener and consists of hydroxyl substituents. The oxidation of D-mannitol results in the formation of D-mannose. Provide the Fischer projection of D-mannitol.
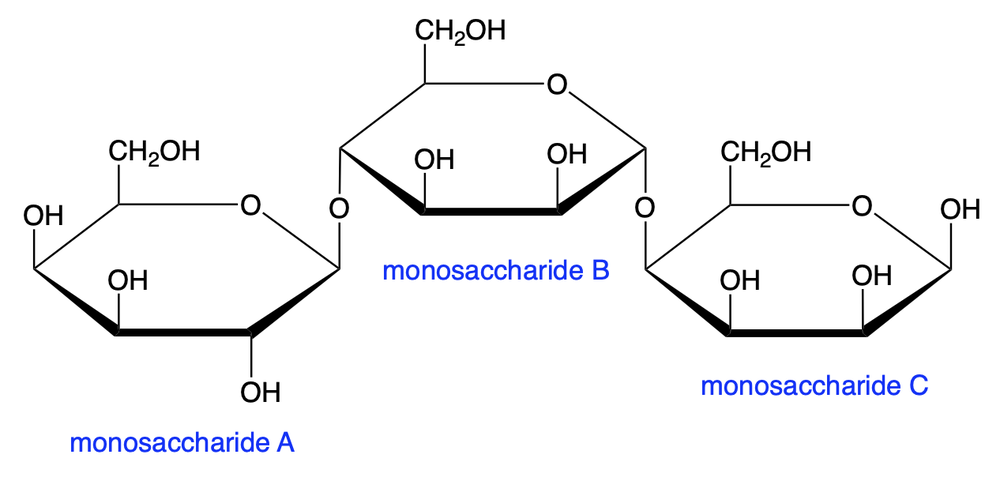
Consider the following trisaccharide:
Determine which carbons form the glycosidic bond between monosaccharide units A and B.
Propose the plausible β structure of the disaccharide that contains two cyclic D-galactose sugars that are linked via the β-1,4 glycosidic bond. Explain why this disaccharide is a reducing sugar, while the sugar below is not reducing sugar.
Lactose is a sugar found in milk and dairy products. Based on its Haworth structure, is lactose considered a monosaccharide, disaccharide, trisaccharide, or polysaccharide?
Which of the four stages of metabolism involves the oxidation of a certain substrate to CO₂ while generating NADH, FADH₂, and a small amount of ATP?
In biological systems, glucose is broken down to produce ATP in a process called cellular respiration. This process is exergonic but does not occur rapidly without specific enzymes present. Why would organisms adopt this strategy?
Determine if Mn2+ acts as a cofactor or coenzyme.
Classify the given as either a cofactor or a coenzyme: Zn2+.
Flavin mononucleotide (FMN) acts as a coenzyme in dehydrogenation reactions. Determine whether FMN is a reducing agent or an oxidizing agent.
Determine which of the following statements is/are true regarding why the breakdown of molecules for energy in the body occurs in several steps.
i. A stepwise breakdown of molecules prevents the production of excessive heat, which could damage cells.
ii. Multiple steps allow for the efficient storage of energy in the form of ATP.
iii. The stepwise process enables the coupling of energetically favorable and unfavorable reactions.
iv. Several steps allow for the generation of more by-products, which are essential for other metabolic processes.
v. A multi-step breakdown increases the risk of harmful intermediates being produced.
Choose the location in the cell where the citric acid cycle occurs.
Which of the following sets of compounds are all four-carbon dicarboxylic acids involved in the citric acid cycle?
Which molecule is responsible for transferring electrons from Complex II to Complex III in the electron transport chain?
Explain why the statement below about oxidative phosphorylation and substrate-level phosphorylation is either TRUE or FALSE.
Oxidative phosphorylation and substrate-level phosphorylation are similar processes that produce ADP but only differ in their mechanisms, efficiency, and oxygen dependence. Substrate-level phosphorylation is the primary ATP generator, while oxidative phosphorylation is just a supplementary process that transforms ATP into ADP.
Determine the ATP yield from the oxidation of six molecules of FADH2.
6 FADH2 → 6 FAD
Indicate whether the following symptoms are for hypoglycemia or hyperglycemia.
(i) high blood sugar level, frequent urination, increased thirst, and low blood pressure
(ii) low blood sugar level, shakiness, sweating, and hunger
What are the initial reactant and final product of the glycolysis process?
Glycolysis and pyruvate oxidation are commonly referred to as the "aerobic oxidation pathway". Is oxygen present in this pathway? Explain.
Which statement is true regarding the transport of glycolysis products to the mitochondrial matrix?
i. ATP is directly used by pyruvate translocase to pump pyruvate into the mitochondrial matrix.
ii. NADH produced during glycolysis is transported directly into the mitochondrial matrix by the pyruvate transporter.
iii. Pyruvate enters the mitochondrial matrix through simple diffusion, powered by the high concentration of pyruvate in the cytoplasm.
iv. Pyruvate is transported into the mitochondrial matrix via a symporter called pyruvate translocase, which co-transports pyruvate along with protons (H⁺).