- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Explain why cellobiose and lactose are considered reducing disaccharides, whereas the disaccharide trehalose is considered non-reducing.
Maltose is a disaccharide, and it has α and β forms. Are these forms enantiomers of each other? Explain your reasoning.
Draw the Haworth structure for a hypothetical disaccharide that is quite similar to β-lactose except it has an α(1→4)−glycosidic bond.
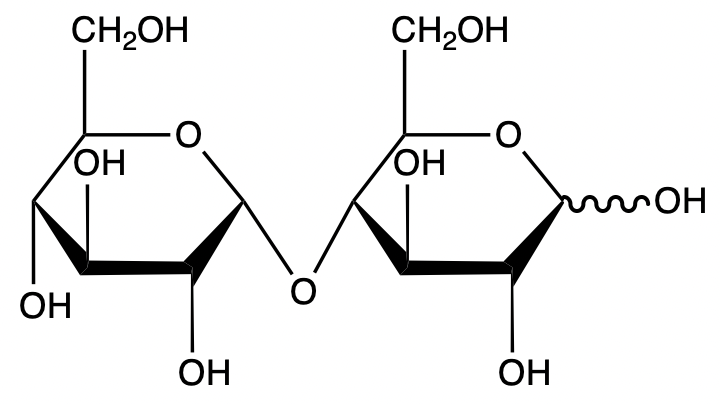
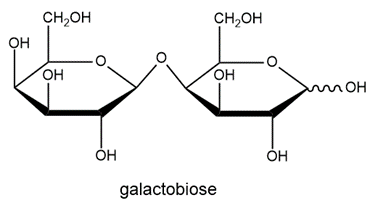
Determine if galactobiose is a reducing sugar. Explain your answer.
During a laboratory experiment, a student hydrolyzed a disaccharide and obtained one molecule of glucose and one molecule of fructose. The experiment notes indicate that the glycosidic linkage is α (1→5). Draw the structure of the disaccharide.
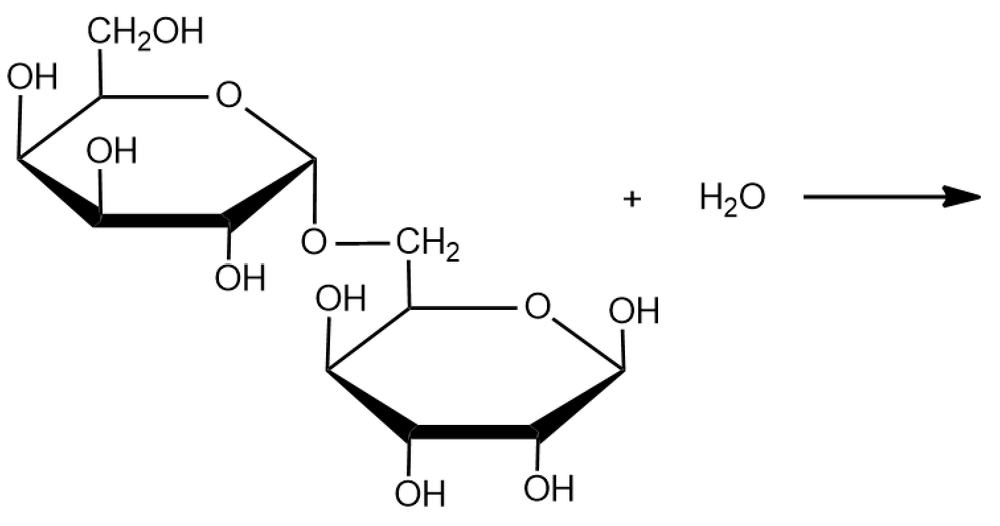
Identify the reaction below as condensation or hydrolysis, and draw the expected products.
Three-letter abbreviation with the glycosidic bond type is a useful technique for representing carbohydrates. For example, sucrose can be denoted as Glcα (1→2) βFru. Using the same concept, draw the structure of the sugar with an abbreviation of Galβ (1→6) Glcα