Here are the essential concepts you must grasp in order to answer the question correctly.
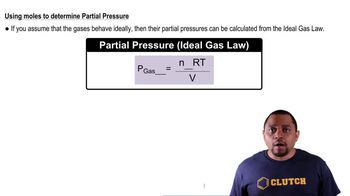
Partial Pressure
Partial pressure refers to the pressure exerted by a single gas in a mixture of gases. It is a crucial concept in understanding gas behavior in the lungs, as each gas contributes to the total pressure of the mixture. In this context, the partial pressures of nitrogen (N2), oxygen (O2), carbon dioxide (CO2), and water vapor (H2O) are used to determine their respective contributions to the overall composition of air in the lungs.
Recommended video:
Dalton's Law: Partial Pressure (Simplified) Concept 2
Percent Composition
Percent composition is a way to express the concentration of a component in a mixture as a percentage of the total. In the context of gases in the lungs, it is calculated by taking the ratio of the partial pressure of each gas to the total pressure of the gas mixture, then multiplying by 100. This allows for a clear understanding of how much of each gas is present relative to the total air composition.
Recommended video:
Dalton's Law of Partial Pressures
Dalton's Law states that in a mixture of non-reacting gases, the total pressure is equal to the sum of the partial pressures of each individual gas. This principle is essential for calculating the total pressure in the lungs and understanding how each gas contributes to the overall pressure. It provides the foundation for determining the percent composition of air based on the measured partial pressures of the gases involved.
Recommended video:
Dalton's Law: Partial Pressure (Simplified) Concept 3