Here are the essential concepts you must grasp in order to answer the question correctly.
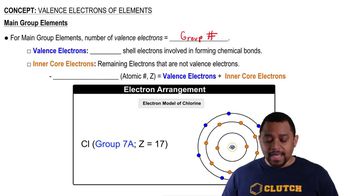
Valence Electrons
Valence electrons are the outermost electrons of an atom and are crucial for determining how an atom can bond with others. In the case of phosphine (PH3), phosphorus has five valence electrons, while each hydrogen atom contributes one, resulting in a total of eight valence electrons for the molecule.
Recommended video:
Valence Electrons of Elements (Simplified) Concept 1
Bonding Pairs
Bonding pairs refer to pairs of electrons that are shared between atoms to form covalent bonds. In phosphine, there are three bonding pairs formed between the phosphorus atom and the three hydrogen atoms, allowing the molecule to maintain its stable structure.
Recommended video:
Lone Pairs
Lone pairs are pairs of valence electrons that are not involved in bonding and remain on the atom. In the case of phosphine, phosphorus has one lone pair of electrons, which influences the molecular geometry and the overall polarity of the molecule.
Recommended video: