Here are the essential concepts you must grasp in order to answer the question correctly.
ATP Hydrolysis
ATP hydrolysis is the process by which adenosine triphosphate (ATP) is broken down into adenosine diphosphate (ADP) and inorganic phosphate (Pi), releasing energy. This energy is often used to drive endergonic reactions, which require an input of energy to proceed. In the context of fatty acid catabolism, ATP hydrolysis provides the necessary energy to link fatty acids to coenzyme-A, facilitating their activation for further metabolic processes.
Recommended video:
Adenosine Triphosphate (ATP) Concept 2
Exergonic vs. Endergonic Reactions
Exergonic reactions are those that release energy, typically occurring spontaneously, while endergonic reactions require an input of energy to proceed. The linking of fatty acids to coenzyme-A is an endergonic process, as it involves the formation of a high-energy thioester bond. The energy from ATP hydrolysis is crucial in making this reaction favorable, effectively coupling the two types of reactions to ensure metabolic efficiency.
Recommended video:
Enantiomers vs Diastereomers Concept 1
Metabolic Coupling
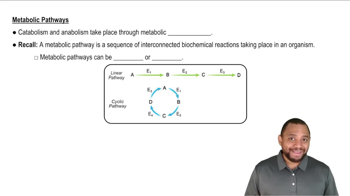
Metabolic coupling refers to the strategy of linking exergonic and endergonic reactions to drive biological processes. In fatty acid CoA synthesis, the hydrolysis of ATP is coupled with the activation of fatty acids, allowing the energy released from ATP breakdown to facilitate the otherwise unfavorable reaction of fatty acid attachment to coenzyme-A. This coupling is a fundamental principle in metabolism, ensuring that energy is efficiently utilized in cellular processes.
Recommended video:
Metabolic Pathways Concept 2
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution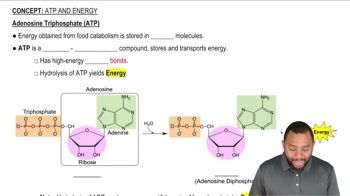


 1:46m
1:46m