Here are the essential concepts you must grasp in order to answer the question correctly.
Ammonium Salts
Ammonium salts are ionic compounds formed from the protonation of amines, where the nitrogen atom carries a positive charge. They typically consist of a positively charged ammonium ion (NH4+) and a negatively charged anion, such as chloride (Cl-). Understanding the structure of ammonium salts is crucial for identifying their properties and reactivity.
Recommended video:
Drawing Ammonium Salts Concept 2
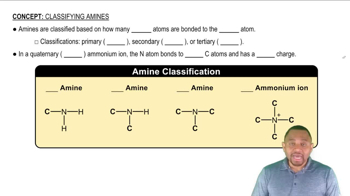
Amines Classification
Amines are classified based on the number of carbon groups attached to the nitrogen atom. Primary amines have one carbon group, secondary amines have two, and tertiary amines have three. This classification affects the properties and behavior of the amine, including its ability to form ammonium salts.
Recommended video:
Amine Classification Concept 1
Nomenclature of Organic Compounds
The nomenclature of organic compounds follows specific rules set by the International Union of Pure and Applied Chemistry (IUPAC). For ammonium salts, the name reflects the structure of the amine and the anion. Understanding these naming conventions is essential for accurately identifying and communicating about chemical compounds.
Recommended video:
Introduction to Organic Chemistry Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 :55m
:55m