Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They use dots to represent electrons and lines to represent bonds. Understanding how to draw and interpret these structures is crucial for identifying molecular geometry and predicting reactivity.
Recommended video:
Lewis Dot Structures: Ions (Simplified) Concept 1
Formal Charge
Formal charge is a concept used to determine the distribution of electrons in a molecule. It is calculated by taking the number of valence electrons in an atom, subtracting the number of non-bonding electrons, and half the number of bonding electrons. A correct Lewis structure minimizes formal charges across the molecule, leading to greater stability.
Recommended video:
Periodic Table: Transition Metals Charges Concept 1
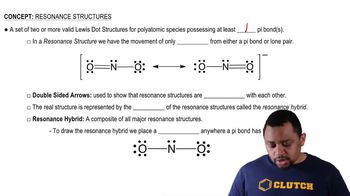
Resonance Structures
Resonance structures are different ways of drawing the same molecule that show the delocalization of electrons. They are used when a single Lewis structure cannot adequately represent a molecule's electron distribution. Understanding resonance is essential for accurately depicting molecules with multiple bonds and predicting their behavior in chemical reactions.
Recommended video:
Resonance Structures (Simplified) Concept 1

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:57m
1:57m