Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Neutralization
Chemical neutralization is a process where an acid and a base react to form water and a salt, effectively reducing the harmful effects of the acid. For instance, sulfuric acid (H₂SO₄) can be neutralized by sodium bicarbonate (NaHCO₃), resulting in less hazardous substances. This method is effective for many chemical spills, but it has limitations when applied to radioactive materials.
Recommended video:
Chemical Reaction: Chemical Change Concept 1
Radioactive Waste
Radioactive waste consists of materials that are radioactive and can emit harmful radiation. Unlike typical chemical spills, radioactive waste poses long-term health risks due to its persistent radioactivity and the potential for contamination. The complexity of managing radioactive waste involves not only neutralization but also containment and disposal, which are more challenging than for non-radioactive chemicals.
Recommended video:
Measuring Radioactivity Concept 1
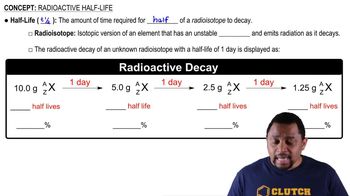
Half-Life and Decay
The half-life of a radioactive substance is the time it takes for half of the radioactive atoms to decay into a more stable form. This decay process can take years, decades, or even longer, making it difficult to 'clean up' radioactive waste in the same way as chemical spills. The long-term nature of radioactive decay means that the waste remains hazardous for extended periods, complicating cleanup efforts.
Recommended video:
Radioactive Half-Life Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:09m
2:09m