Here are the essential concepts you must grasp in order to answer the question correctly.
Isoelectronic Species
Isoelectronic species are atoms or ions that have the same number of electrons and, therefore, the same electronic configuration. In this question, Se<sup>2-</sup>, Sr<sup>2+</sup>, Rb<sup>+</sup>, and Br<sup>-</sup> all have 36 electrons, making them isoelectronic. Understanding this concept is crucial for comparing their atomic radii, as they share similar electron arrangements.
Recommended video:
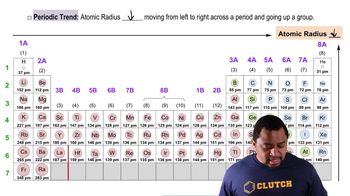
Atomic Radius Trends
Atomic radius refers to the size of an atom, typically measured from the nucleus to the outermost electron shell. In general, atomic radius increases down a group in the periodic table due to the addition of electron shells, while it decreases across a period due to increased nuclear charge. For isoelectronic species, the effective nuclear charge experienced by the electrons influences their size, with more protons pulling electrons closer and resulting in a smaller radius.
Recommended video:
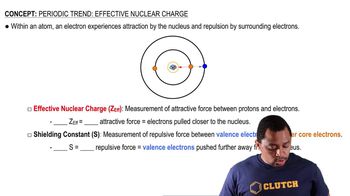
Effective Nuclear Charge (Z_eff)
Effective nuclear charge (Z_eff) is the net positive charge experienced by an electron in a multi-electron atom, accounting for both the total nuclear charge and the shielding effect of inner electrons. In the context of isoelectronic species, the greater the number of protons in the nucleus, the higher the Z_eff, leading to a smaller atomic radius. Thus, comparing the atomic radii of the given ions involves analyzing their respective nuclear charges and how they affect the size of the electron cloud.
Recommended video:
 Verified step by step guidance
Verified step by step guidance