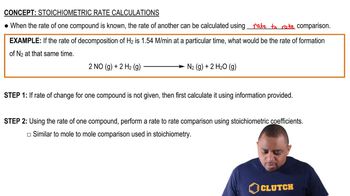
Textbook Question
The distance from Earth to the sun is 1.5×108 km. Find the number of crests in a light wave of frequency 1.0×1014 s –1 traveling from the sun to Earth.
2153
views
1
rank



The distance from Earth to the sun is 1.5×108 km. Find the number of crests in a light wave of frequency 1.0×1014 s –1 traveling from the sun to Earth.
A 5.00-mL ampule of a 0.100-M solution of naphthalene in hexane is excited with a flash of light. The naphthalene emits 12.3 J of energy at an average wavelength of 349 nm. What percentage of the naphthalene molecules emitted a photon?
A laser produces 20.0 mW of red light. In 30.0 minutes, the laser emits 1.15×1020 photons. What is the wavelength of the laser?