Here are the essential concepts you must grasp in order to answer the question correctly.
Nomenclature of Ionic Compounds
Ionic compounds are named based on the cation and anion they contain. The cation is named first, followed by the anion. For metals that can form more than one charge, such as tin (Sn), the charge must be indicated using Roman numerals in parentheses. In the case of SnO2, tin is the cation and oxide is the anion.
Recommended video:
Oxide Ions
Oxide ions are negatively charged ions formed when oxygen gains two electrons, resulting in O2-. In ionic compounds, oxide typically pairs with metals to form stable compounds. Understanding the charge of oxide is crucial for correctly naming compounds like SnO2, where the oxide ion's charge influences the overall charge balance.
Recommended video:
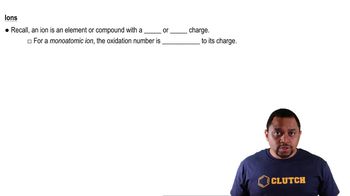
Oxidation Numbers in Ions
Determining Oxidation States
The oxidation state of an element in a compound indicates the degree of oxidation or reduction it has undergone. In SnO2, the oxidation state of oxygen is -2. To maintain charge neutrality, the oxidation state of tin must be +4, leading to the name tin(IV) oxide. Recognizing how to calculate oxidation states is essential for proper nomenclature.
Recommended video:
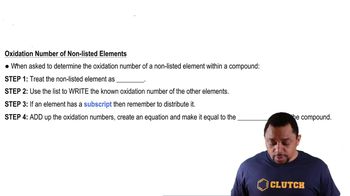
Determining Oxidation Numbers
 Verified step by step guidance
Verified step by step guidance