Textbook Question
Write the formula for each ionic compound. f. iron(II) phosphate
962
views
 Verified step by step guidance
Verified step by step guidance
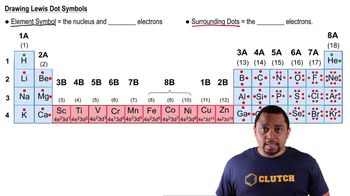
Write the formula for each ionic compound. f. iron(II) phosphate
Write the name from the formula or the formula from the name for each hydrated ionic compound.
a. CoSO4•7 H2O
b. iridium(III) bromide tetrahydrate
c. Mg(BrO3)2•6 H2O
d. potassium carbonate dihydrate
Write the name from the formula or the formula from the name for each hydrated ionic compound.
a. cobalt(II) phosphate octahydrate
b. BeCl2•2 H2O
c. chromium(III) phosphate trihydrate
d. LiNO2•H2O
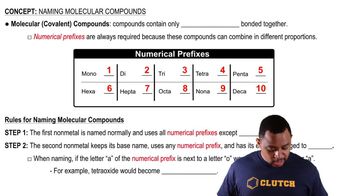
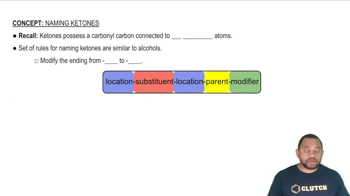
Name each molecular compound. a. SO3 c. BrF5
Name each molecular compound. b. SO2
Name each molecular compound. d. NO