Textbook Question
Classify each element as atomic or molecular. a. bromine b. titanium c. oxygen d. potassium
 Verified step by step guidance
Verified step by step guidance

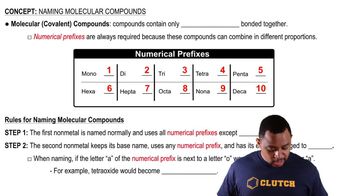

Classify each element as atomic or molecular. a. bromine b. titanium c. oxygen d. potassium
Identify the elements that have molecules as their basic units. a. nitrogen b. argon c. hydrogen d. helium
Classify each compound as ionic or molecular. a. CO2 b. NiCl2 c. Nal d. PCl3
Based on the molecular views, classify each substance as an atomic element, a molecular element, an ionic compound, or a molecular compound.
Based on the molecular views, classify each substance as an atomic element, a molecular element, an ionic compound, or a molecular compound.
Write a formula for the ionic compound that forms between each pair of elements. a. silver and chlorine b. sodium and sulfur c. aluminum and sulfur d. potassium and chlorine