Here are the essential concepts you must grasp in order to answer the question correctly.
Alkanes
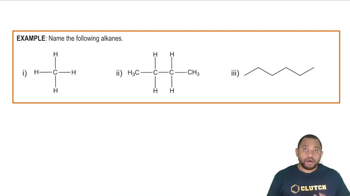
Alkanes are a class of hydrocarbons characterized by single bonds between carbon atoms, following the general formula CnH2n+2. They are saturated compounds, meaning they contain the maximum number of hydrogen atoms per carbon atom. Alkanes can be straight-chain or branched, and their names are derived from the number of carbon atoms they contain, such as methane (1 carbon), ethane (2 carbons), and propane (3 carbons).
Recommended video:
Nomenclature
Nomenclature in organic chemistry refers to the systematic naming of chemical compounds. For alkanes, the International Union of Pure and Applied Chemistry (IUPAC) provides rules for naming based on the number of carbon atoms and the structure of the molecule. The names typically end with the suffix '-ane' to indicate that they are alkanes, and prefixes like 'meth-', 'eth-', 'prop-', etc., denote the number of carbons.
Recommended video:
Isomerism
Isomerism is the phenomenon where compounds with the same molecular formula exhibit different structural arrangements. In alkanes, structural isomers can occur when carbon atoms are arranged in different ways, leading to distinct compounds with unique properties. For example, butane (C4H10) has two isomers: n-butane (a straight chain) and isobutane (a branched chain), which have different boiling points and chemical behaviors.
Recommended video:
Isomerism in Coordination Complexes Example
 Verified step by step guidance
Verified step by step guidance