Here are the essential concepts you must grasp in order to answer the question correctly.
Alkanes
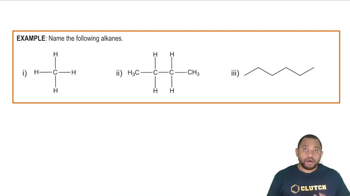
Alkanes are saturated hydrocarbons consisting only of carbon (C) and hydrogen (H) atoms, connected by single bonds. They follow the general formula CnH2n+2, where n is the number of carbon atoms. Alkanes are characterized by their relatively low reactivity and are commonly found in natural gas and petroleum.
Recommended video:
IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) nomenclature provides a systematic way to name chemical compounds. For alkanes, names are derived from the longest continuous carbon chain, with prefixes indicating the presence of substituents. In the case of '4,7-diethyl-2,2-dimethylnonane', the numbers indicate the positions of the substituents on the main chain.
Recommended video:
Structural Representation
Structural representation of organic compounds illustrates the arrangement of atoms within a molecule. For alkanes, this can be depicted using line-angle formulas, where lines represent bonds between carbon atoms. Understanding how to draw these structures is essential for visualizing molecular geometry and predicting chemical behavior.
Recommended video:
 Verified step by step guidance
Verified step by step guidance