What products are obtained in the electrolysis of a molten mixture of KI and KBr?
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction: Ag+(aq) + e– → Ag(s) What mass of silver would plate onto the cathode if a current of 6.8 A flowed through the cell for 72 min?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
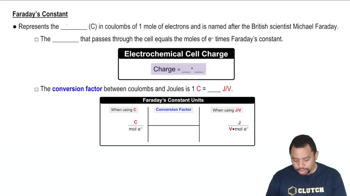
Key Concepts
Electrolysis

Faraday's Laws of Electrolysis
Current and Charge

Write equations for the half-reactions that occur at the anode and cathode for the electrolysis of each aqueous solution. a. Ni(NO3)2(aq)
Make a sketch of an electrolysis cell that electroplates copper onto other metal surfaces. Label the anode and the cathode and indicate the reactions that occur at each.
A major source of sodium metal is the electrolysis of molten sodium chloride. What magnitude of current produces 1.0 kg of sodium metal in 1 hour?
Consider the reaction shown here occurring at 25°C. Cr(s) + Cd2+(aq) → Cr2+(aq) + Cd(s) Determine E°cell, K, and ∆G°rxn for the reaction and complete the table.
[Cd2+] [Cr2+] Q Ecell 𝚫Grxn
1.00 1.00
1.00 1.00 × 10-5
1.00 × 10-5 1.00
4.18 × 10-4 1.00
Consider the unbalanced redox reaction: Cr2O72-(aq) + Cu(s) → Cr3+(aq) + Cu2+(aq) Balance the equation and determine the volume of a 0.850 M K2Cr2O7 solution required to completely react with 5.25 g of Cu.
