Predict the conditions (high temperature, low temperature, all temperatures, or no temperatures) under which each reaction is spontaneous. a. H2O(g) → H2O(l) b. CO2(s) → CO2(g) c. H2(g) → 2 H(g) d. 2 NO2(g) → 2 NO(g) + O2(g) (endothermic)
Ch.19 - Free Energy & Thermodynamics
Chapter 19, Problem 55a
Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning. a. NH3(g); Ne(g); SO2(g); CH3CH2OH(g); He(g) c. CH4(g); CF4(g); CCl4(g)
 Verified step by step guidance
Verified step by step guidance1
Identify the phase of each substance. All substances are in the gaseous phase, so we will compare their molar entropies based on molecular complexity and atomic size.
Consider the atomic size and molecular complexity. Generally, larger atoms and more complex molecules have higher entropies.
Compare the noble gases first: He(g) and Ne(g). Helium, being smaller, will have a lower entropy than neon.
Compare the molecular gases: NH_3(g), SO_2(g), and CH_3CH_2OH(g). Consider the number of atoms and the complexity of the molecules. More atoms and more complex structures typically lead to higher entropy.
Rank the substances from lowest to highest entropy based on the analysis: He(g) < Ne(g) < NH_3(g) < SO_2(g) < CH_3CH_2OH(g).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
0m:0sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Standard Molar Entropy
Standard molar entropy (S°) is a measure of the amount of disorder or randomness in a system at standard conditions (1 bar, 25°C). It reflects the number of accessible microstates for a substance, with higher values indicating greater disorder. Entropy increases with molecular complexity, molecular weight, and the number of atoms in a molecule.
Recommended video:
Guided course

Standard Molar Entropy
Phase of Matter
The phase of matter significantly influences entropy. Gases generally have higher entropies than liquids and solids due to their greater freedom of movement and higher number of microstates. Among gases, larger and more complex molecules tend to have higher entropies than smaller, simpler ones, as they can occupy more spatial configurations.
Recommended video:
Guided course
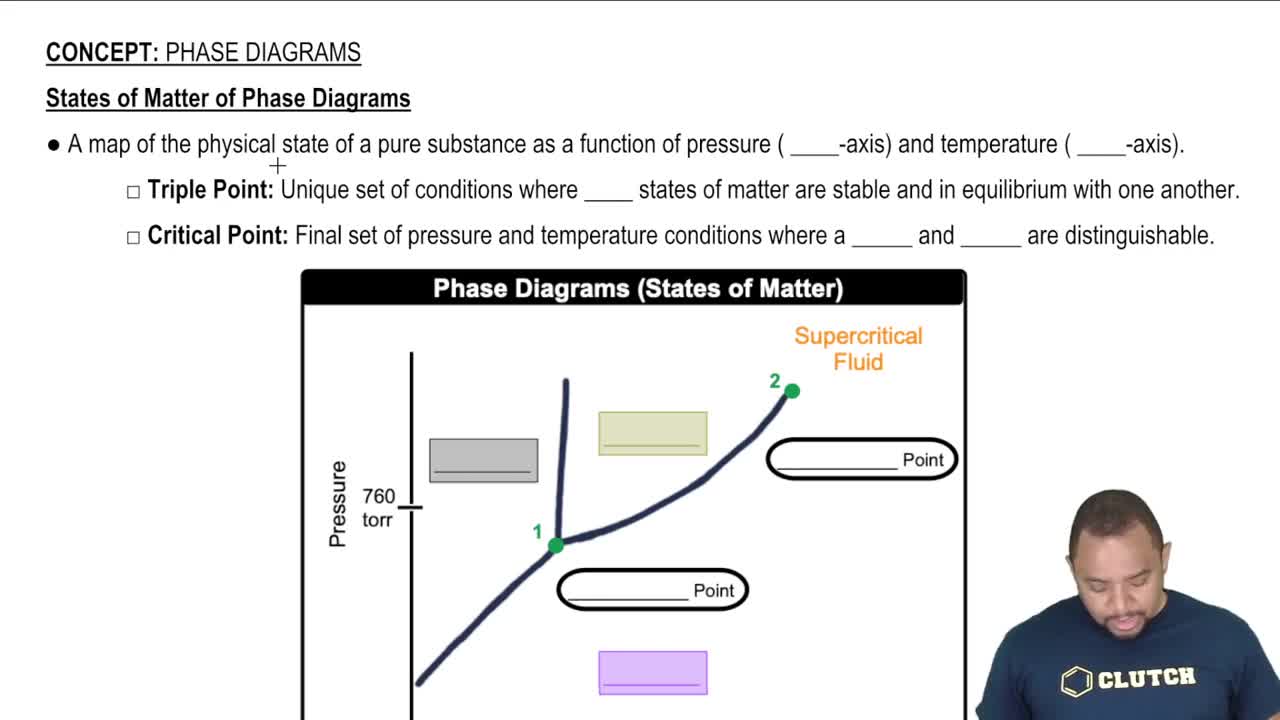
States of Matter of Phase Diagrams
Molecular Structure and Complexity
The molecular structure and complexity of a substance affect its entropy. Larger molecules with more atoms and functional groups can vibrate and rotate in more ways, leading to higher entropy. For example, comparing NH3 and CH3CH2OH, the latter has a more complex structure and thus a higher standard molar entropy due to increased molecular interactions and configurations.
Recommended video:
Guided course
Complex Ions Example
Related Practice
Textbook Question
1571
views
Textbook Question
How does the molar entropy of a substance change with increasing temperature?
889
views
Textbook Question
For each pair of substances, choose the one that you expect to have the higher standard molar entropy (S°) at 25 °C. Explain your choices. a. CO(g); CO2(g) b. CH3OH(l); CH3OH(g) c. Ar(g); CO2(g) d. CH4(g); SiH4(g) e. NO2(g); CH3CH2CH3(g) f. NaBr(s); NaBr(aq)
1639
views
Textbook Question
Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning. b. H2O(s); H2O(l); H2O(g)
561
views
Textbook Question
Rank each set of substances in order of increasing standard molar entropy (S°). Explain your reasoning. a. I2(g); F2(g); Br2(g); Cl2(g) b. H2O(g); H2O2(g); H2S(g) c. C(s, graphite); C(s, diamond); C(s, amorphous)
738
views
Open Question
Use data from Appendix IIB to calculate ΔSrxn ° for each of the reactions. In each case, try to rationalize the sign of ΔSrxn ° . a. C2H4(g) + H2(g) → C2H6(g)
